2 professionals
Bioderma Congress Reports WCPD 2025
Bioderma Congress Reports WCPD 2025
Get access to exclusive dermatological services to increase your professionnal knowledge: +500 pathology visuals, clinical cases, expert videos
Benefit from valuable features: audio listening, materials to be shared with your patients
Stay informed about the upcoming events and webinars, latest scientific publications and product innovations
Already have an account? login now
Reports written by Dr Maria Florencia Martinez (Pediatrician & Pediatric dermatologist, Argentina) and Dr Paola Stefano (Paediatric Dermatologist, Argentina).

Related topics
Reports written by Dr Maria Florencia Martinez (Pediatrician & Pediatric dermatologist, Argentina)
Speaker: Enrique Salvador Rivas Zaldivar (Guatemala)
Less frequent but important systemic diseases: Dermatomyositis and Langerhans cell histiocytosis
| Feature | Psoriasis | Seborrheic Dermatitis |
|---|---|---|
| Scale | Silvery-white, thick | Oily, yellowish, or dry |
| Plaque | Well-demarcated | Poorly defined |
| Erythema | Prominent, especially beyond hairline | Less well-defined, more subtle |
| Itching | Present | Also present → may cause scratching lesions |
| Retroauricular Involvement | Common | Less frequent |
| Peripheral Scaling | More in SD, scaling follows hair shafts |
Seen in SD, rarely in psoriasis |
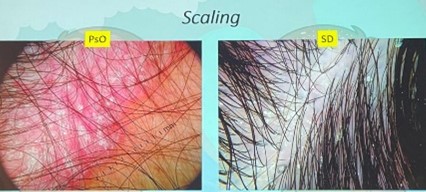
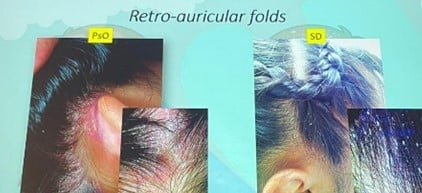
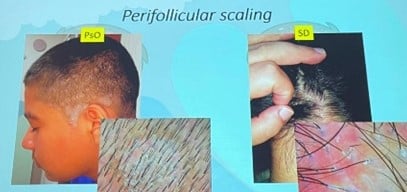
Note: Both diseases may present erythema and scale, making clinical diagnosis difficult in isolation.
Even histology can be inconclusive in some overlapping presentations.
That’s where dermatoscopy (trichoscopy) becomes essential.
Vascular Patterns – The Key Differentiator:
| Dermatoscopic Finding | Psoriasis | Seborrheic Dermatitis |
|---|---|---|
| Vessel Type | Red dots, glomerular vessels, twisted red loops |
Arborizing blurry vessels, atypical patterns |
| Localization | Superficial dermal capillaries |
Deeper and less defined vessels |
| Scale Appearance | Thick, dry, White | Yellowish, greasy |
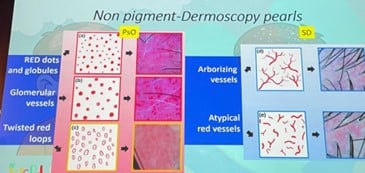
(Bruni et al., 2021)
Contributing factor: Malassezia spp. overgrowth in all age groups
References:
- Bruni F, et al. Clinical and trichoscopic features in various forms of scalp psoriasis. J Eur Acad Dermatol Venereol. 2021 Sep;35(9):1830-1837.
- Silverberg NB. Scalp hyperkeratosis in children with skin of color: diagnostic and therapeutic considerations. Cutis. 2015 Apr;95(4):199-204
- Waśkiel-Burnat A et al. Differential diagnosis of red scalp: the importance of trichoscopy, Clinical and Experimental Dermatology, 2024, Setp; 49 (9): 961–968
- Kim GW et al. Dermoscopy can be useful in differentiating scalp psoriasis from seborrhoeic dermatitis. Br J Dermatol. 2011 Mar;164(3):652-6
Speaker: Arturo Lopez Yañez Blanco (Mexico)
The speaker began with a general overview of tinea capitis emphasizing that it's a fungal scalp infection mainly affecting children (98%), caused by dermatophytes of the Trichophyton and Microsporum genera.
Epidemiology & Clinical Forms
The etiological agents vary by region: M canis, T tonsurans, M audouinii, T mentagrophytes, T verrucosum.
Two main clinical forms:
|
Inflammatory tinea capitis
|
Non-inflammatory tinea capitis |
| Tender plaques with pustules and crust |
Single or multiple hair loss patches
|
| Painful abscess | Scaly patches |
| Lynphadenopathy | Pruritus |
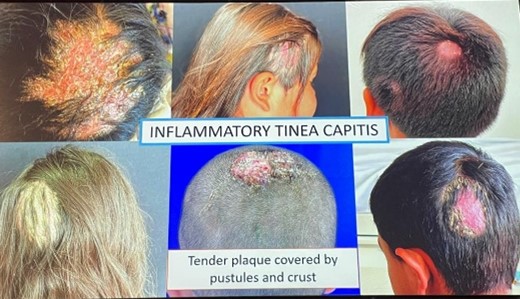
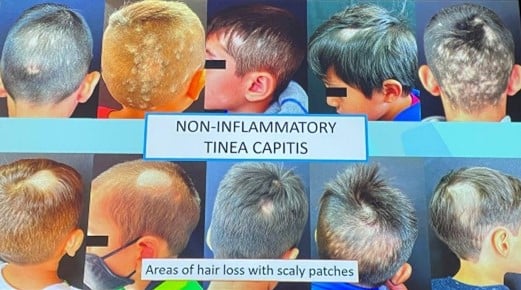
Diagnostic Tools
| Inflammatory tinea capitis | Non-inflammatory tinea capitis |
| Follicular pustules | Corkscrew hairs |
| Crusts | White sheaths |
| Perifollicular scaling | Perifollicular and diffuse scaling |
| Linear vessels | Black dots |
| Erythema | Morse code-like hairs |
| Comma hairs /Zigzag hairs | |

Triad of signs (hair loss, scaly patches, peritoneal involvement) = high predictive value (95%).
Differential Diagnosis:
Treatment Recommendations
Common parental questions addressed:
Tracing contact in school is essential.
Introduction
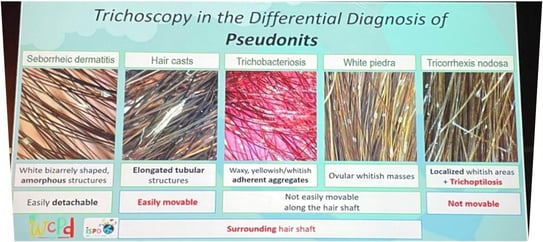
Trichoscopic Differences
True nits: brown with oval heads.
Pseudonits: translucent, grayish, black tips.
Clinical Signs
Treatment
No major new treatments. Conventional therapy remains effective.
Education for parents about lice behavior and contagion is essential.
THERAPY PRESCRIPTION
PERMETRIN
LOTION 1%. Apply and washep up the hair after 10 min, repeat in 1 week
MALATHION
LOTION 0,5%. Apply and washep up the hair after 8-12hs, repeat in 1 week
BENZYL ALCOHOL
LOTION 5%. Apply and washep up the hair after 10 min, repeat in 1 week
IVERMECTIN
LOTION 0,5%. Apply and washep up her hair after 10 min, repeat in 1 week
ORAL TABLETS: 0,2 mg/k/d 2 days, repeat in 1 week
TMS
ORAL: 10 mg/k/d 3 days BID, repeat in 1 week
References:
Speaker: Cecilia Navarro Tuculet (Argentina)
Introduction:
Types of Telogen Effluvium:
Common Triggers of Telogen Effluvium in Children:
Prevalence in Pediatric Population:
Diagnosis and Evaluation:
Differential Diagnosis:
Management:
Take-Home Messages:
References:
Speaker: Arturo Lopez Yañez Blanco (Mexico)
Definition:
Epidemiology:
Pathophysiology:
Clinical Features:
Diagnostic Findings:
Definition:
Epidemiology:
Pathophysiology:
Clinical Features:
Diagnostic Findings:
Differential Diagnosis:

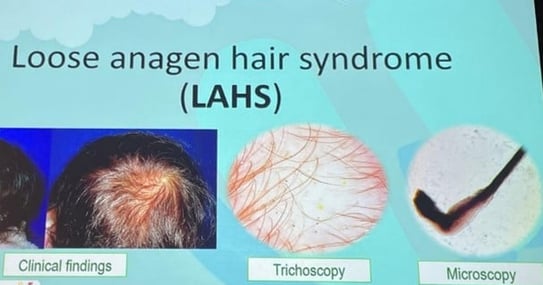
| Feature | SAS | LAHS |
|---|---|---|
| Age at diagnosis | 2–6 years | 6–10 years |
| Sex predominance | Female | Female > Male |
| Hair appearance | Short (<10cm), sparse, fine |
Fine, short (>20cm), easily pulled |
| Pull test | Negative or mildly positive |
Positive, painless |
| Trichoscopy | Normal | Rectangular/circular black dots, dirty dots |
| Trichogram | ↑ Telogen hairs | Anagen hairs (70%) distorted bulbs and roots |
| Microscopy | Normal hair shafts | Disrupted inner root sheath |
| Pathophysiology | Shortened anagen phase | Poor anchoring of anagen hairs |
| Genetic component | WNT10A mutation (40%) | Keratin gene defects |
| Associated syndromes | Scleroderma, micronychia, etc. | Noonan syndrome, TRPS |
| Prognosis | Benign, improves with age | |
| Minoxidil use | Possible if psychosocially indicated | |
References:
Cranwell WC, et al. Loose anagen hair syndrome: Treatment with systemic minoxidil characterised by marked hair colour change. Australas J Dermatol. 2018;59(4):e286-e287.
Lemes LR, et al. Topical and oral minoxidil for hair disorders in pediatric patients: What do we know so far? Dermatol Ther. 2020;33(6):e13950.
Starace M, et al Short anagen syndrome: A case series and algorithm for diagnosis. Pediatr Dermatol. 2021;38(5):1157-1161
Speaker: Miguel Marti (Argentina)
Common symptoms:
Clinical signs:
Clinical presentation:
| Treatment Modality | |
|---|---|
| Topical corticosteroids | First-line therapy |
| Intralesional corticosteroids | Effective in localized disease |
| Systemic corticosteroids | In more severe cases |
| Hydroxychloroquine, methotrexate | Reserved for refractory cases |
Clinical characteristics:
References:
Speaker: Lizet Rojano Fritz (Colombia)
| Patient | 8-year-old female. No relevant personal or familiar medical history |
|---|---|
| Initial Dx | Alopecia areata (Dec 2024) |
| Treatment | Clobetasol + Prednisone → No response |
| Culture | Positive for Microsporum canis |
| Treatment 1 | Terbinafine x 3 months |
| Follow-up | Persistent central alopecia → repeat trichoscopy: black dots, broken hairs |
| Culture | Still positive |
| Treatment 2 | Griseofulvin x 6 months |
| Observation | Vertex with poor regrowth, trichoptilosis, fine hairs |
| Final Dx | Tinea capitis + Trichotillomania (Patient admitted to hair pulling at night) |
Key Point:
Consider dual diagnoses in pediatric alopecia. Always confirm tinea capitis via culture.
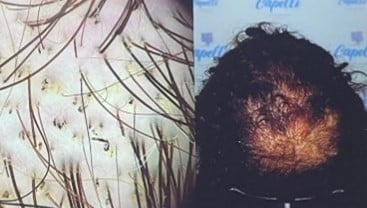
Trichoscopy – Findings by Condition
| Feature | Trichotillomania | Tinea Capitis |
|---|---|---|
| Broken hairs Black dots |
✅✅ | ✅ |
| Trichoptilosis Flame hairs Tulip hairs V sign Microexclamation hairs |
✅ | ❌ |
| Yellow dots | ✅/❌ | ❌ |
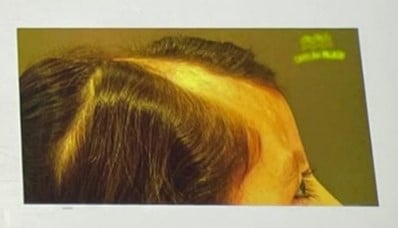
| Patient | 8-year-old female |
|---|---|
| History | 5 years of linear facial plaque from vertex to forehead/eyebrow |
| Symptoms | Social withdrawal, no medical history |
| Biopsy | Linear morphea |
| Trichoscopy | Loss of Follicular openings, broken hairs, black dots, pink areas, pili torti. |
| MRI | Thinning of subcutaneous tissue (scalp & facial region) |
| Autoimmune panel | Negative |
| Treatment | Hair transplant after 4 years of inactive lesion |
| Result | Successful regrowth, improved self-esteem |
Key Point:
Hair transplantation can be effective in inactive morphea with no ongoing inflammation.
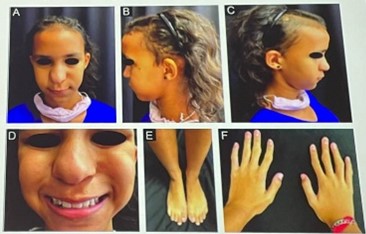
| Patient | 15-year-old female |
|---|---|
| Hx | Congenital hypotrichosis |
| Trichoscopy | Normal |
| Trichogram | Dystrophic anagen bulbs |
| Phenotypic Features | Bulbous nose, cone shaped epiphyses hands and feet, maxillary prognathism, thin upper lip |
| Genetic Test | Deletion at chromosome 8q24.12 |
| Dx | TRPS Type I |
| Treatment | Topical minoxidil → Good hair density at 6 months |
Key Point:
In congenital hypotrichosis, always assess for syndromic features and genetic testing.
References:
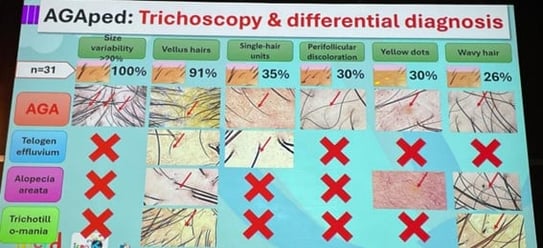
Speaker: Luis Sanchez Dueñas (Mexico)
| Feature | Frequency |
|---|---|
| Hair diameter variability | 100% |
| Vellus hairs | High |
| Yellow dots | Occasional |
| Peripilar sign | Common |
| Wavy hairs | Present |
| Focal atrophy | Rare |
| Indication for Referral | Recommended Action |
|---|---|
| Pre-pubertal onset | Endocrine referral |
| Male with female pattern hair loss |
Hormonal panel |
| Female with signs of hyperandrogenism |
Hormonal panel |
| Suggested labs | Testosterone, DHEA-S, SHBG, LH/FSH, Vitamin D, Glucose, Insulin |
Differential Diagnosis: Telogen effluvium, Alopecia areata, trichotillomania
First-line (Both sexes):
If partial response:
Adjuncts:
Advanced therapy (if poor response):
| FEMALE | MALE | |
| PREPUBERAL | TOPICAL MINOXIDIL 2% | |
| PUBERAL | TOPICAL MINOXIDIL 5% | |
| PARCIAL IMPROVEMENT | Oral minoxidil 0,25-0,5 mg/d Topical Finasteride Topical/Oral Espironolactone |
Oral minoxidil 1-2,5 mg/d Topical Finasteride |
| LIMITED IMPROVEMENT | Oral Finasteride 2,5 mg/d |
Oral Finasteride 1 mg/d |
| Medication | Event | Action Taken |
|---|---|---|
| Oral Finasteride | Gynecomastia (Male) | Stopped → Reversible |
| Oral Minoxidil | Trichomegaly (Excess lashes) | Dose reduction |
References:
Speaker: Sonia Ocampo-Garza (Mexico)
| Group | Clinical Pattern | First-line Treatment | Alternative Options |
|---|---|---|---|
| 1 | <50% scalp, mild activity, with regrowth | Topical corticosteroids + Minoxidil | Anthralin, intralesional triamcinolone, dexamethasone pulses |
| 2 | >50% scalp, high activity/resistance/ ophiacean pattern | Oral dexamethasone + Topical clobetasol or minoxidil | Methotrexate, Cyclosporine, Hydroxychloroquine |
| 3 | Totalis/Universalis | Contact immunotherapy ± Topical steroids | JAK inhibitors, oral steroids, methotrexate |
Systemic Corticosteroids
| Type | Dose | Notes |
|---|---|---|
| Topical | 2–5% | Widely used |
| Oral | 0.5 mg/day | 71% improved in case series (hypertrichosis most common AE) |
Agents:
Protocol:
Adverse Events:
| Drug | JAK Target | Pediatric Use | Notes |
|---|---|---|---|
| Tofacitinib | Pan-JAK | Off-label | 87% response in 31 patients |
| Baricitinib | JAK1/2 | Off-label | 68% SALT reduction |
| Ritlecitinib | JAK3/TEC | FDA-approved ≥12 years | SALT <20 in 25–50% at 48 wks |
| Extent | Activity | Primary Approach | Secondary Options |
|---|---|---|---|
| Localized | Active | Topical corticosteroids + Minoxidil | Anthralin |
| Inactive | Immunotherapy | ||
| Extensive | Active | Systemic corticosteroids, JAK inhibitors (Ritlecitinib: approved 12 yo), Immunosuppressants | |
| Inactive | Immunotherapy or JAK inhibitors, Systemic corticosteroids |
References:
Speaker: Lawrence Eichenfield (USA)
Despite recent advances in systemic agents, topical care remains foundational in AD management, even in patients on systemic treatment.
Topical therapy includes:
A critical point raised is that new topical agents are typically studied against vehicles, rather than active comparators like corticosteroids, and usually as monotherapy, which doesn’t reflect real-world practice where combination therapy is common.
The speaker's core message to patients is to aim for long-term disease control, defined as:
Topical corticosteroids remain first-line treatment in many cases.
Steroid phobia is discussed with a notable shift from concern about atrophy to a growing belief in "topical steroid addiction" (TSA). The speaker highlights the video “Skin on Fire” as an example of this messaging. (https://www.youtube.com/watch?v=GuaBbsL1qKA)
While topical steroid withdrawal syndrome (TSW) is a recognized phenomenon, particularly in adults (rare in pediatric population) with long-term use, the concept of widespread topical steroid addiction lacks evidence.
A Swedish internet survey of self-identified TSW patients (recruited via Facebook) reported:
Clinical trial data may overstate perceived effectiveness. In a study of BID mid-potency corticosteroids for 4 weeks:
These results reflect modest short-term efficacy even for traditional agents, reinforcing the need for novel therapies.
1. Topical Ruxolitinib (JAK1/2 Inhibitor)
Pediatric Data: In 2–12-year-olds, ~56% achieved clear/almost clear with BID application.
2. Tapinarof (Aryl Hydrocarbon Receptor Agonist)
Long-term data:
3. Crisaborole (Topical PDE4 Inhibitor)
4. Roflumilast (PDE4 Inhibitor)
| Formulation | Indication | Age | Notes |
|---|---|---|---|
| 0.3% cream | Psoriasis | ≥6 | First approval |
| 0.3% foam | Seborrheic dermatitis | Adult | Newer formulation |
| 0.15% cream | Atopic dermatitis | ≥6 | Recently approved |
| 0.05% cream | AD (age 2–5 study) | 2–5 | Not yet approved |
5. Delgocitinib (Pan-JAK Inhibitor)
Current Topical Options
Cost and Access Challenges
Combination Therapy and Real-World Practice
References:
Speaker: Carsten Flohr (United Kingdom)
While advanced therapies in atopic dermatitis (AD) are gaining prominence, conventional systemic treatments remain foundational. This presentation addresses the strategic use of both conventional and newer systemic treatments, framed through clinical evidence and a detailed case study.
Atopic dermatitis is characterized by a chronic itch-scratch cycle that perpetuates inflammation. Exacerbating factors must be managed before initiating systemic therapy:
Effective management should include:
Methotrexate
Cyclosporine
Following suboptimal or complicated responses to conventional agents, advanced therapies may be introduced.
Dupilumab
JAK Inhibitors (e.g., baricitinib, upadacitinib, abrocitinib)
Network meta-analyses (NMAs) provide comparative efficacy and safety data for systemic treatments. One application of this is the clinical decision-support platform [EczemaTherapies.com], designed to:
For patients with multifaceted disease courses or limitations to monotherapy, combination regimens may be necessary.
Case Study Overview:
A patient with severe, early-onset AD (EASI >50 at age 10) demonstrated:
This treatment approach is now published in Pediatric Dermatology as an example of advanced therapeutic integration.
Optimal management of atopic dermatitis requires a nuanced balance between guideline-based strategies, emerging evidence, and real-world complexities. The therapeutic landscape is expanding rapidly, and with it, the opportunity to tailor interventions based on disease severity, comorbidities, and patient preferences—always with a multidisciplinary approach at the center.
References:
Speaker: Elaine Siegfried (USA)
Checklist Prior to Initiating Systemic Therapy:
| Consideration | Description |
|---|---|
| Endotype | E.g., classical AD, contact dermatitis, psoriasis/psoriasiform overlap |
| Non Atopic Comorbidities | Systemic comorbidities, Sleep disruption, HSV (herpes incognito), recurrent otitis media, pneumonia |
| Atopic Comorbidities | Especially ocular (risk for dupilumab-induced conjunctivitis) |
| Corticosteroid Exposure | Topical and systemic |
| Psychosocial Factors | Adherence barriers, anxiety, needle phobia |
| BMI & Development | Consider nutritional status |
| Family History | Atopy, autoimmune, immunodeficiency |
| Access and Cost | May influence therapy selection |
Recognized Subtypes:
| Biomarker | Interpretation |
|---|---|
| Total/Specific IgE | Degree of atopic sensitization |
| Eosinophil Count | HES if >1500 × 3 |
| Albumin, Total Protein, Globulins | Nutritional and immunologic markers |
| ANA, Histone | Possible correlation with anti-drug antibody formation |
| Vitamin D | Often deficient; screen for rickets |
| Celiac Screening | Especially in failure to thrive |
| Immunologic Panel | Recurrent infections warrant screening for PID |
Clinical Metrics:
| Response | Criteria |
|---|---|
| Optimal | IGA 0 (clear) |
| Acceptable | IGA 1 (almost clear), stable mild disease |
| Failure | No meaningful improvement (e.g., <2-point IGA drop) |
| Cause | Note |
|---|---|
| Psoriasiform Skewing | Poor response to Type 2 inhibitors |
| Allergic Contact Dermatitis | Difficult to detect, patch testing limited |
| Non-Adherence | Often underestimated |
| Needle Phobia | Impacts biologic use. Management: Home-based behavioral techniques Topical anesthetics Pharmacologic support Mental health referral |
| Anti-Drug Antibodies | Not well studied in AD yet |
| Undiagnosed Infection | May be unmasked post-immunomodulation |
| Primary Immunodeficiency | Often missed without full immune workup |
| Hypersensitivity to Excipients | E.g., polysorbates (noted in most biologics) Polysorbate 80 - Dupilumab, tralokinumab Polysorbate 188 - Nemolizumab |
| Clinical Clue | Consideration |
|---|---|
| Persistent inflammation | Increase topical steroid or add systemic |
| Injection refusal | Oral JAKi or systemic alternatives |
| Excipient reaction | Switch class if possible (not always feasible) |
| Long-term JAKi risk | Transition back to biologic when stable |
| Poor response to dupilumab | Transition to JAKi (e.g., upadacitinib) |
| Allergic contact dermatitis phenotype | Patch testing, methotrexate |
| Suspected anti-drug antibody | Switch to a different class (e.g., JAKi) |
| Psoriasiform phenotype | IL-12/23 blockade (e.g., ustekinumab) may be more effective |
| Adherence issues | Simpler regimens, oral options |
References:
Speaker: Amy S. Paller (USA)
1. Topical Therapies: Evolving Options
| Agent | Status | Age Range | Notes |
|---|---|---|---|
| Roflumilast | Emerging | Likely expanding | Minimal stinging/burning |
| Ruxolitinib | Approved | ≥12 years | Potential for younger use in future |
| Tapinarof | Approved | ≥2 years | Low irritation profile |
2. Novel Targets and Pathways
3. Drug Delivery Innovations
| Method | Limitation |
|---|---|
| Microneedles | Not feasible for high-volume biologics |
| Needle-free injectors | Volume limitations (e.g., dupilumab = 2mL) |
| Sensor-triggered feedback devices | Potential for behavioral intervention |
4. Systemic Therapies: Present and Future
5. Itch as a Therapeutic Frontier
| Pathway | Agent or Target |
|---|---|
| IL-31 | Nemolizumab |
| IL-4/IL-13 | Dupilumab, JAK inhibitors |
| Substance P | Failed trials so far |
| Proteases (e.g., V8 protease) | Antiplatelet drugs as novel topicals |
| PAR2 activation | Calocrine inhibition under study |
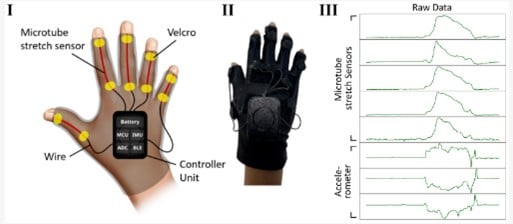
6. Remote Monitoring & Digital Trials
Remote Clinical Trials:
Feasible with:
Wearable Technology:
Tracks:
Examples:
8. Next-Gen Technologies:
| Technique | Application |
|---|---|
| Proteomics (5,000 proteins) | Serum analysis from small blood volumes |
| Single-cell transcriptomics | High-resolution skin data |
| Microbiome swabbing | Genomics and proteomics |
| Detergent-based epidermal swabbing | Non-invasive immune profiling |
| Area | Future Outlook |
|---|---|
| Therapeutics | More non-steroidal topicals, pain-free biologics, small molecule inhibitors |
| Monitoring | Objective wearable sensors, real-time feedback |
| Access & Equity | Need for cost reduction and global availability |
| Clinical Trials | Move toward remote, tech-enabled designs |
References:
Speaker: Jane Bellet (USA)
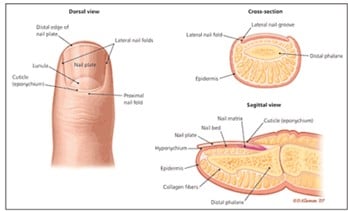
1. Nail Anatomy Review:
2. When to Biopsy:
3. Pediatric Considerations:
4. Pre-op Planning:
5. Anesthesia Techniques:
6. Tourniquet Options: bloodless field
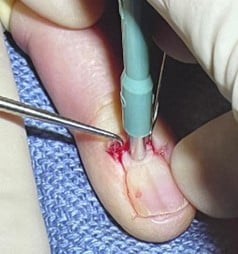
7. Common Procedures:
A. Punch Biopsy:
B. Partial Proximal Nail Avulsion:
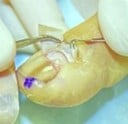
C. Nail Matrix Shave Excision:
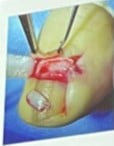
8. Specimen Handling:
9. Tourniquet Removal:
"Most important part of your day."
10. Bandaging Tips:
11. Post-op Care:
12. Special Pediatric Considerations:
13. When Not to Operate:
References:
Speaker: Robert Silverman (USA)
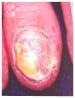
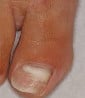
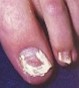
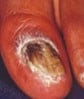
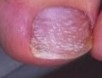
Dermoscopy clues:
Topical:
Systemic:
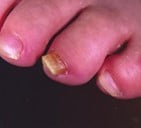
References:
Speaker: Judith Dominguez Cherit (Mexico)
Longitudinal melanonychia (LM) in pediatric patients is a diagnostic challenge. While well-studied in adults, limited data is available in children. The condition may be caused by either melanocytic activation (racial/ethnic or functional) or proliferation (e.g., nevi, melanoma).
| Feature | Adults | Children |
|---|---|---|
| Ethnic melanonychia | Common, especially in darker skin types | Rare, even in darker skin types |
| Melanoma risk | LM may be an early sign | Extremely rare |
| Diagnostic criteria | Well-established | Often unreliable when applied to children |
| Histology appearance | Predictive | May mimic melanoma, even when benign |
BRAFV600E, HMB-45, and PNL2 staining may assist in differentiating between benign and malignant melanocytic lesions. Negative BRAFV600E findings are typically associated with benign nevi.
References:
Speaker: Maria Sol Dia (Argentina)
Two clinical contexts:
Dermatologic associations:
Systemic associations:
Clinical Subtypes
| Subtype | Description |
|---|---|
| Opaque Trachyonychia | Most common and severe. Nails appear rough, thin, with longitudinal ridging, and loss of shine. |
| Shiny Trachyonychia | Intermittent inflammation with glassy appearance, light reflection, and fine ridging. |
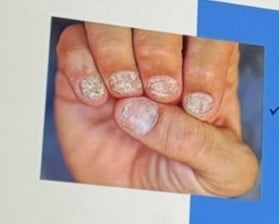
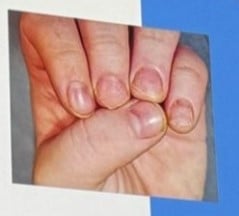
Common findings in both types:
Clinical Approach:
Tools:
Biopsy:
| Approach | Indication |
|---|---|
| Observation | Most pediatric cases resolve spontaneously within 6 years |
| Topical treatments | Cosmetic concern or associated dermatoses |
| Intralesional corticosteroids | Refractory localized disease (painful, may be distressing) |
| Systemic therapy | Severe or associated with psoriasis, alopecia areata, etc. |
Common topical agents:
Systemic agents in severe/associated cases:
5-year-old boy with progressive nail roughness, previously treated with various topicals without improvement.
4-year-old girl with a history of atopic dermatitis
References:
Speaker: Lourdes Navarro Campoamor (Spain)
Diagnostic Importance of Nails in Children
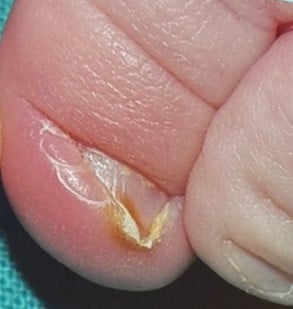
Congenital hypertrophy of the lateral nail fold
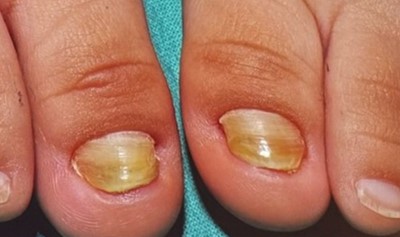
Congenital malalignment of the toenail
Congenital anonychia
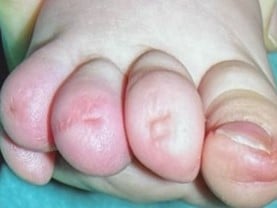
Iso-Kikuchi Syndrome
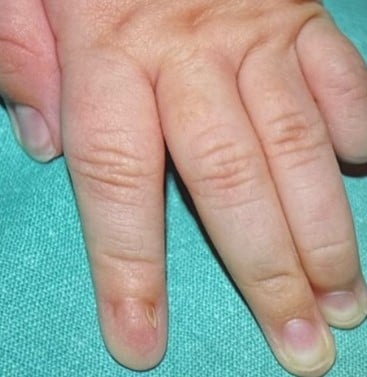
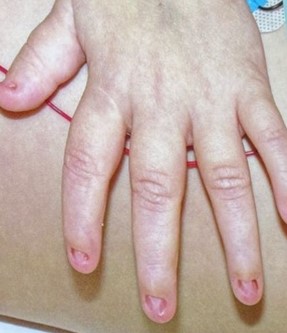
Nail-Patella Syndrome
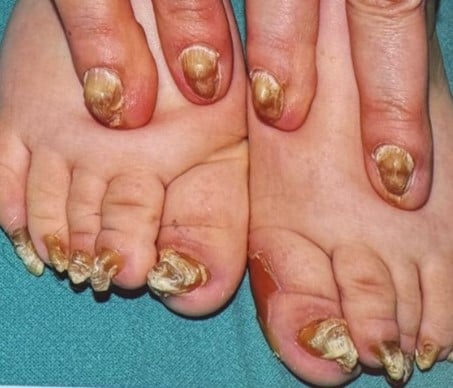
Pachyonychia Congenita
Tuberous Sclerosis Complex
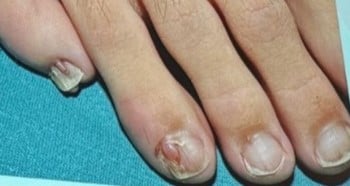
Darier Disease
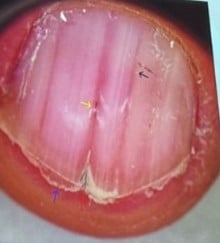
Melanonychia & Fibromas
| Finding | Possible Associated Conditions |
|---|---|
| Triangular lúnula | Nail Patella Syndrome |
| Micronychia | Iso-Kikuchi, Nail Patella Syndrome |
| Anonychia/ Micronychia index fingers |
Iso-Kikuchi |
| Thickened toenails | Pachyonychia congenita |
| Periungual fibromas | Tuberous sclerosis complex |
| Longitudinal red/white streaks, distal wedge-shaped subungual keratosis, V shaped notch |
Darier disease |
References:
Speaker: Kelly Cordoro (USA)
Speaker: Henry W. Lim (USA)
Photodermatoses are skin disorders caused or aggravated by sunlight.
Categories:
Focus on:
1. Polymorphous Light Eruption (PMLE)
Clinical Features:
Variants:
Diagnosis:
Pathophysiology:
Management:
2. Actinic Prurigo
Treatment:
3. Hydroa Vacciniforme (HV) & HV-like Lymphoproliferative Disease
| Classical Hydroa Vacciniforme (HV) | HV-like Lymphoproliferative Disease |
|
|---|---|---|
| Frequency | Rare | Severe variant |
| Age of Onset | Childhood | Childhood or adolescence |
| Skin Lesions | Papulovesicular eruptions healing with vacciniform scars | Lesions on sun-exposed and non-exposed areas |
| Systemic Symptoms | Absent | Facial edema, fever, lymphadenopathy |
| Association with EBV |
Strong | Present |
| Photosensitivity | May show UVA sensitivity on phototesting | Not specifically reported |
| Prognosis | Variable, treatment is challenging | Risk of progression to lymphoma |
| Main Treatment | Photoprotection | Onco-hematological management |
4. Solar Urticaria
Clinical Tip:
Always evaluate patients immediately post-exposure/testing — waiting 24 hours can miss the diagnosis.
Treatment:
5. Erythropoietic Protoporphyria (EPP) & X-linked Protoporphyria (XLP)
Pathophysiology:
Clinical Clues:
Rationale: Blocks porphyrin accumulation upstream in the pathway.
| Condition / Type | Typical Age of Onset | Clinical Morphology | Diagnosis | Treatment / Management | Key Points |
|---|---|---|---|---|---|
| Polymorphous Light Eruption (PMLE) | Children, adolescents (more common in teens) | Papules, vesicles; morphology varies with skin phototype | Clinical (based on history and morphology); phototesting often normal | Photoprotection, topical corticosteroids, narrowband UVB, oral prednisone (for flares) | Delayed onset post-sun exposure; improves with progressive sun exposure ("hardening") |
| Actinic Prurigo | Childhood | Cheilitis, conjunctivitis, lichenified papules on sun-exposed skin | Clinical evaluation | Photoprotection, topical/oral corticosteroids, thalidomide, UVB, dupilumab (experimental) | Latin America; intense sun exposure |
| Classic Hydroa Vacciniforme (HV) | Children | Papules, vesicles, crusting; vacciniform scarring | Clinical, EBV association possible |
Photoprotection, symptomatic care | Permanent scarring; severe phototoxic response |
| HV-like Lymphoproliferative Disease | Children and adolescents | HV-like lesions on both sun-exposed and non-exposed skin; facial edema, fever, lymphadenopathy | Clinical + biopsy + hematologic | Oncology referral, close monitoring | May progress to lymphoma (~10%); high mortality (~40%); more severe in Latin America |
| Solar Urticaria | Children, adolescents, adults |
Wheals in sun-exposed areas; rapid onset (minutes) | Clinical + immediate post-exposure evaluation | Antihistamines, omalizumab, photoprotection | Triggered even by visible light; resolves quickly but recurs with re-exposure |
| Erythropoietic Protoporphyria (EPP / XLP) | Early childhood |
Pain, burning, erythema shortly after sun exposure; chronic changes on knuckles and face | Elevated protoporphyrin IX levels; genetic testing | Afamelanotide (implant), Derzimegalone (oral), glycine transporter inhibitors (in trials), photoprotection | Major therapeutic advances; improves quality of life significantly |
References:
Speaker: Jean Bologna (USA)
| Term Used | Notes |
|---|---|
| Speckled Lentiginous Nevus (SLN) | Preferred by some clinicians; emphasizes clinical pattern. |
| Nevus Spilus | More commonly used in literature (PubMed ratio 2:1); literally means “stained spot.” |
| Other terms | Excessive terminology noted; need for standardization emphasized. |
| Feature | Description |
|---|---|
| Size | Typically 3–4 cm in diameter |
| Base | Café-au-lait macule-like background |
| Overlay | Lentigines, junctional nevi, compound nevi |
| Evolution | More "dots" appear with age; may initially be subtle at birth |
| Distribution Patterns | May follow Blaschko's lines or block-like patterns; not always checkerboard |
| Syndrome/Entity | Association with SLN |
|---|---|
| PPK (Phacomatosis pigmentokeratotica) | Papular SLN |
| PPV (Phacomatosis pigmentovascularis) | Macular SLN |
| Neurocutaneous melanocytosis | Can occur in patients with medium-sized CMN arising within SLN |
| Rhabdomyosarcoma | Reported in a patient with Spitz nevi within SLN (rare) |
| Noonan syndrome (RASopathies) | Mentioned as having overlapping features |
| Argument | Evidence |
|---|---|
| Congenital lesion | Lesions follow embryological patterns (e.g., Blaschko's lines); hybrid presentations support this. Evidence supports congenital origin |
| Apparent "acquisition" | Hypopigmented base may be subtle at birth; speckling appears later, leading to misclassification |
References:
Speaker: Aniza Giacaman Contreras (Spain)
| Structure | Description |
|---|---|
| Dots | Small pinpoint areas of pigmentation. |
| Globules | Larger than dots, may vary in shape; correspond to melanocytic nests. |
| Lines | Can form distinct dermoscopic patterns such as networks or branches. |
| Pigmented Network | Reflects melanin in keratinocytes at the dermoepidermal junction. |
| Cobblestone Pattern | Globules clustered in a mosaic-like pattern, commonly seen in CMN. |
| Target Structures | Globules within a network with central dots; may suggest melanocyte apoptosis. |
| Blue/Gray Dots | Indicate melanocyte apoptosis or dermal involvement. |
| Vessels | Presence may vary by lesion evolution; can include coarse vessels. |
| Hypertrichosis | Often associated with congenital nevi, increasing with age. |
| Location | Common Patterns |
|---|---|
| Head | Homogeneous pattern |
| Trunk | Predominantly globular pattern |
| Extremities | Reticular or mixed globular-reticular pattern |
| Palms/soles | Parallel ridge or furrow patterns, “peas-in-a-pod” appearance |
Classic Globular Pattern
Mixed Network and Globules
Changes Over Time
Acral Areas (Palms, Soles)
Nail Matrix Involvement
| Scenario | Clinical Course |
|---|---|
| Nodules stable over years | Often benign if no suspicious dermoscopic features are present. |
| Cobblestone globules without atypia | Favor benign course; continued monitoring recommended. |
| Nodules with blue coloration | May raise concern; histology may still be benign. |
| Rapidly growing nodules in short time | Biopsy warranted to rule out malignancy (e.g., atypical Spitz tumor). |
References:
Speaker: Julia Schaeffer (USA)
| Term | Definition |
|---|---|
| Congenital melanocytic nevus (CMN) | Present at birth or within the first few weeks of life |
| Size-based classification | Based on projected adult size: small (<1.5 cm), medium (1.5–20 cm), large (>20 cm), giant (>40 cm) |
| Targeted CMN / Congenital-like nevi | Lesions appearing within the first 2–3 years; histologically and molecularly similar to true congenital nevi |
Common Dermoscopic Patterns by Location
| Scalp |
|
| Trunk |
|
| Acral |
|
| Nails |
|
| Feature | Interpretation |
|---|---|
| Globular pattern | Typical of CMN; benign feature |
| Reticular/blue-gray globules | Seen in deeper melanocyte proliferation; not always concerning |
| Target globules | Globules within network; associated with melanocytic activity |
| Fibrillar brush pattern (nails) | Common in children; benign |
| Vascular features | Can appear over time; not always suggestive of melanoma |
| Risk Estimate | Evidence |
|---|---|
| < 0.5–1% lifetime risk | Systematic reviews & cohort data (e.g., 15-year risk: ~0.007%) |
| Melanoma onset | Typically post-pubertal; rare in early childhood |
Clinical Monitoring
Indications for Excision
| Indication | Reason |
|---|---|
| Cosmetic/psychosocial | Most common modern indication, especially facial lesions |
| Functional concerns | Lesions near joints, eyelids, etc. |
| Changes suggestive of malignancy | Focal color, texture, or vascular changes |
| Parental anxiety | When psychological impact is high and persistent |
Excision Techniques
Laser Therapy
Topical therapies
Timing of Excision:
| Lesion Type | Management Note |
|---|---|
| Halo Nevi | Benign |
| Nodular components | Require thorough history and monitoring |
| Scalp lesions | Tends to fade |
| Acral/Nail lesions | Recognize benign dermoscopic patterns |
- No intervention needed unless atypical features present
- Avoid premature biopsy
- Biopsy only if concerned
References:
Speaker: Fatima Giusti (Argentina)
The speaker presented their experience with digital dermoscopy in pediatric dermatology, emphasizing the need for well-defined clinical indications. The presentation was based on a retrospective study conducted at a tertiary hospital with expertise in both digital dermoscopy and pediatric care.
Demographics & Skin Type
Follow-up & Usage
Risk Factors & Clinical Syndromes
Notably, the prevalence of atypical mole syndrome was significantly higher than in published pediatric cohorts, suggesting potential benefit from structured digital follow-up.
Dermoscopic Patterns
Indications for Digital Dermoscopy
Excision & Histopathology
Findings support the role of digital dermoscopy in avoiding unnecessary surgery while still identifying clinically significant lesions.
Nevus Count
Genetic Conditions
References:
Speaker: Elena Hawryluk (USA)
| Size Category | Lifetime Melanoma Risk |
|---|---|
| Small/Medium CMN | <1% Risk mostly post-puberty |
| Large CMN | ~2% |
| Giant CMN with satellites | 6–15% Highest risk group |
Definition & Risk:
Imaging Strategy:
| Criteria (vary across guidelines) |
|---|
| >3 cm CMN or ≥25 CMN |
| >1 CMN regardless of size |
| Giant CMN, multiple medium CMN |
| ≥4 CMN associated with abnormal CNS findings (Mass General cohort) |
MRI screening guidelines remain non-standardized and are resource-dependent across regions. Shared decision-making with parents is critical.
References:
Speaker: Veronica Kinsler (United Kingdom)
This presentation provided an in-depth review of the genetic mechanisms underlying congenital melanocytic nevi (CMN), focusing on mosaic mutations, especially NRAS, BRAF, and BRAF fusion genes—and their clinical significance. The talk also explored the emerging role of targeted therapy, particularly MEK inhibitors, in treating patients with problematic CMN phenotypes.
| Gene | Prevalence | Mutation Type | Clinical Features |
|---|---|---|---|
| NRAS | Most common | Missense mutations |
Found in skin, CNS, and tumors; consistent across tissues |
| BRAF | Less common | Missense mutations | Often associated with numerous soft dermal nodules |
| BRAF fusions | Newly recognized subgroup | Gene fusion (varied partners) | Often associated with multinodular, treatment-resistant CMN |
| Key Characteristics |
|---|
|
References:
Speaker: Marius Rademaker (New Zealand)
| Rosacea | Perioral Dermatitis (POD) | |
|---|---|---|
| Common Presentation | Facial erythema, flushing, papules/pustules | Papules around mouth, nose, and eyes |
| Flushing | Nearly universal | Usually absent |
| Ocular Involvement | Common (Blepharitis, conjunctivitis) |
Not typical |
| Comedones | Absent | |
| Demodicosis | Possible | |
| Symptoms Description | Burning or stinging | Painful itch |
| Worsened by topical corticosteroids | Yes | |
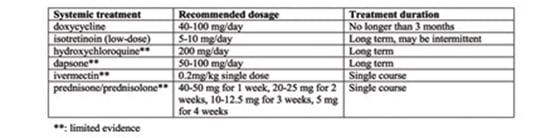
General Principles
Topical Therapies
Systemic Therapies
Antibiotics: Use with Caution
Isotretinoin (off-label)
Oral Ivermectin
Resume:
References:
Speaker: Irene Lara Corrales (Canada)
1. Steroid-Induced
2. Targeted Therapies (e.g., EGFR & MEK inhibitors)
3. JAK Inhibitor-Induced (JAK-ny)
1. Pityrosporum (Malassezia) Folliculitis
2. Gram-Negative (Hot Tub) Folliculitis
| Feature | Acne | Acneiform Eruption |
|---|---|---|
| Comedones | Present | Absent |
| Onset | Gradual | Sudden, within days–weeks |
| Distribution | Variable | Symmetric, folliculocentric |
| Triggers | Endogenous | Drug or infectious exposure |
References:
Speaker: Maria Lertora (Argentina)
1. Traditional Acne Pathogenesis Expanded
2. Microbiome Diversity in Healthy vs. Acne Skin
3. Microbial Interactions
4. External Influences on the Microbiome
Commonly Studied Probiotic Strains:
1. Oral Probiotics
2. Biome-Friendly Topical Care
3. Personalized Acne Management
References:
Speaker: Agnes Schwieger (Switzerland)
Hormonal Background: Acne is often an external manifestation of hormonal shifts, primarily associated with two developmental pathways:
Of these, adrenal androgens—specifically the activation of the HPA axis—are thought to play a more prominent role in early-onset acne. This activation begins around age six and is known as adrenarche. It leads to increased production of DHEAS, a weak androgen that can be converted into more potent forms, promoting sebaceous gland growth and sebum production. This hormonal activity contributes to acne, body odor, and the development of pubic hair.
Clinical Focus: The lecture centers on infantile acne (starting after 8 weeks of age) and mid-childhood acne (ages 1–7), noting that neonatal acne is rare and often misdiagnosed (commonly confused with neonatal cephalic pustulosis).
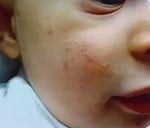
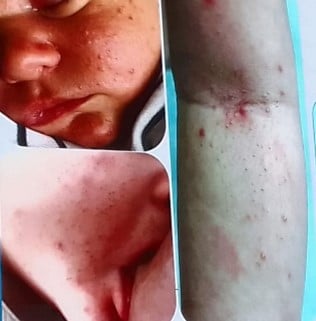
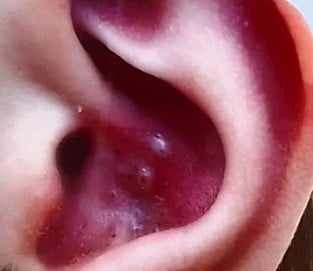
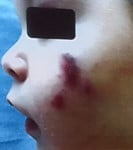
Clinical Pearls:
| Comedones – Papules | Azelaic acid, topical retinoids + BP |
| Pustules | + topical ATB/ oral ATB (erythromycin) |
| Nodules - Pseudocysts | oral ATB, oral retinoid (0,3-0,5 mg/k/d) +/- steroid |
Childhood acne, especially in the infantile and mid-childhood periods, warrants thoughtful evaluation. While most cases are benign and hormonally driven, it’s important to rule out systemic or endocrine disorders. With appropriate management, including topical and systemic treatments when needed, these children can achieve excellent outcomes without long-term consequences.
References:
Speaker: Karen Chernoff (USA)
Acne in females may serve as an isolated dermatologic condition or as a potential clinical marker of hyperandrogenism—a state of androgen excess due to ovarian, adrenal, or peripheral sources. Dermatologists play a key role in recognizing clinical signs of hormonal imbalances and initiating appropriate evaluations or referrals.
Hyperandrogenism results from increased androgen production or peripheral conversion. The pituitary gland stimulates the ovaries and adrenal glands, leading to testosterone production, which is converted to dihydrotestosterone (DHT) by 5-alpha-reductase, resulting in clinical manifestations such as acne.
Core Screening Tests
Total & Free Testosterone (if available via LC-MS/MS)
Additional Labs:
Summary Take-Home Points
References:
Speaker: Andrea Zaenglein (USA)
1. Benzoyl Peroxide in Acne Treatment
2. Isotretinoin and Sexual Dysfunction
References:
Speaker: Pearl Kwong (USA)
Examples of Positive Use:
| Case/Trend | Description |
|---|---|
| Foot peel complication | 15-year-old used a trendy foot mask post-injury → developed painful “blue” pus drainage. |
| Do-it-yourself filler | Attempted self-injection based on online tutorial. |
| Tea tree oil misuse | Use in prepubertal boys → gynecomastia. |
| Topical steroid misuse | Pregnant woman applied clobetasol for acne, worsening her condition. |
| Portal abuse | Patients send photos via EMR portal expecting remote diagnosis (e.g., no visible scale or swelling, yet labeled as such by patient). |
| Life coach over medical care | Delay in treating infected ulcerated hemangioma due to parental reliance on unqualified life coach. |
| Failed acne treatment | Long-time patient sought online acne treatments, worsened before returning. |
| Molluscum home remedy | Application of unknown topical remedy caused extreme hyperpigmentation and skin damage. |
| Snail mucin trend | Popular product among teens despite lack of robust evidence. |
Speaker: Veronica Kinsler (United Kingdom)
Study Example – Arteriovenous Malformations (AVMs):
Findings:
siRNA Strategy:
References:
Speaker: Pierre Vabres (France)
The presentation addressed a newly defined group of mosaic disorders characterized not only by clinical features but also by their underlying molecular causes. These disorders, previously described using various clinical terms (e.g., hypomelanosis of Ito, pigmentary mosaicism), are now increasingly classified based on genetic alterations and signaling pathway involvement.
1. mTOR-related Hypomelanosis of Ito
| Gene | Syndrome/Phenotype | Features |
|---|---|---|
| AKT3 | MPPH syndrome | Hemimegalencephaly, PMG |
| PTEN | Cowden/hamartoma syndromes | Macrocephaly |
| PIK3CA | MCAP syndrome | Capillary malformations, overgrowth |
| PIK3R2 | MPPH syndrome | White matter anomalies |
2. RhoA-related Mosaic Syndrome
3. GNA13-related Mosaic Syndrome
Conclusion: Novel somatic variants in mTOR, RhoA, and GNA13 highlight the importance of exome sequencing in skin tissue and contribute to reclassifying these disorders within genetically defined entities.
References:
Speaker: Gianluca Tadini (Italy)
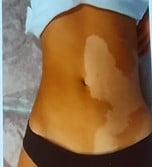
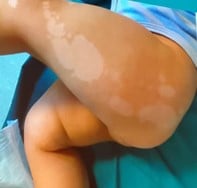
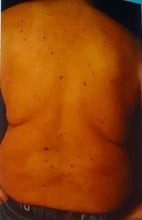
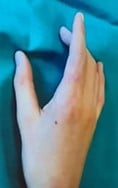
References:
Speaker: Nicole Knoepfel (Switzerland)
Methodology:
| Gene | # of Patients | Clinical Notes | Allele Load | Blood |
|---|---|---|---|---|
| GNAS | 3 | One case developed hyperthyroidism at age 12 | Very low | Negative |
| NRAS | 2 | Fair-skinned child with subtle pigmentation, one with nevi | Low | Negative |
| PTPN11 | 1 | Patchy pigmentation, no nevi, pinkish tone | N/A | Negative |
| BRAF | 1 | Segmental pigmentation with nevi | N/A | Negative |
| Chromosomal Mosaicism | 1 | Epilepsy due to carnitine deficiency, 5q gain in skin | N/A | Negative |
Speaker: Aniza Giacaman Contreras (Spain)
| Lesion | Features | Clinical Notes |
|---|---|---|
| Hypomelanotic macules | Oval, ash-leaf, polygonal, confetti | Often first sign; requires differentiation from vitiligo, nevus anemicus/acromicus |
| Poliosis | White hair patch | Rare but diagnostic |
| Facial angiofibromas | Pink-red papules on central face, sparing upper lip | May resemble acne or rosacea; dermoscopy may show Demodex tails in rosacea |
| Fibrocephalic plaque | Yellowish to brown plaques, scalp or forehead | May be congenital or arise in early infancy |
| Oral fibromas & dental pits | Papules on gums and enamel pitting | Mandates dental referral |
| Peri-/subungual fibromas | Skin-colored or pink papules; nail groove formation | More common on feet; may also appear post-trauma in healthy individuals |
| Shagreen patch | Connective tissue hamartoma with "orange peel" texture | Usually on lumbosacral area |
| UNCOMMON PRESENTATION OF TSC | ||
| Sclerotic bone lesions | Detected radiologically | Important differential: osteoblastic metastases |
|
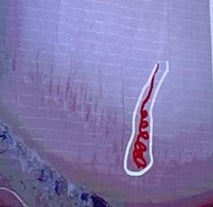
Red comets
|
Corkscrew-shaped periungual vessels with whitish halo | Seen in adult women with TSC |
| White epidermal nevi (WEN) | Early-life white hyperkeratotic papules | May precede hypomelanotic macules; potential early marker of TSC |
| Folliculocystic and collagen hamartoma |
Congenital scalp tumor; comedo-like openings with tufted hairs | Histology: follicular cysts, collagen bundles, and ruptured follicles |
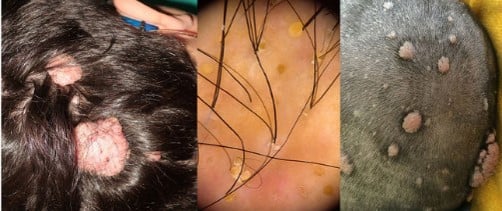
TSC exhibits a broad phenotypic spectrum, and while many signs are cutaneous, several are subtle or underrecognized. Early detection, thorough examination, and multidisciplinary care are essential for effective management.
References:
Speaker: Maria Rosa Cordisco (USA)
1. Capillary Malformations (CM)
Syndromes Associated with Capillary Malformations
| Syndrome | Key Features | Imaging Findings | Treatment Considerations |
|---|---|---|---|
| MCAP Syndrome | Capillary malformation on upper lip/philtrum, megalencephaly, digital anomalies | Polymicrogyria, corpus callosum anomalies, Chiari I | Early neurologic referral, genetic counseling |
| Sturge-Weber Syndrome (SWS) | Facial CM (bilateral V-shaped), leptomeningeal angiomatosis, ocular involvement (glaucoma) | MRI with contrast, eye exams, follow-up imaging for seizures | Early MRI (repeat if negative at <8 weeks), ophthalmologic follow-up |
| GNA11 Mutation | Extensive, reticulated CM, CNS involvement, less aggressive than SWS | MRI brain, less severe neurological involvement | Treat seizures, monitor for glaucoma |
Management of Sturge-Weber Syndrome
Multifocal Capillary Malformations
2. Arteriovenous Malformations (AVMs)
| Key Features | Occurrence | Treatment |
|---|---|---|
| AVMs in CNS | 90% in CNS | Surgery, embolization |
| CAMS (Cerebrofacial Arteriovenous Metameric Syndrome) | Unilateral, metameric distribution | Neurosurgical embolization |
3. Hemangiomas with CNS Involvement
| Type | CNS Risk | Key Associations |
|---|---|---|
| Multifocal hemangiomas | Rare CNS lesions (may be asymptomatic) | Screen for visceral involvement |
| Segmental hemangiomas | Risk of PHACE Syndrome | Requires CNS imaging |
PHACE Syndrome
Imaging: MRI head/neck/chest; echocardiogram
Monitoring: Early MRI for stroke risk, less frequent imaging after first year
References:
Speaker: Maria Teresa Garcia Romero (Mexico)
| Pattern Type | Description | Associated Manifestations |
|---|---|---|
| Blaschko’s Lines | Narrow or broad lines following a linear distribution | Found in both melanocytic and non-melanocytic mosaicism |
| Block-like Patterns | Well-defined, localized patches | Associated with neurocutaneous disorders and systemic involvement |
| Lateralization | Unilateral involvement, often localized | Mosaic may manifest only on one side of the body |
| Sash-like Patterns | Diagonal stripes across the body | Typically associated with segmental mosaicism |
| Large Patches | Larger, well-circumscribed areas | Seen in conditions like hypomelanosis of Ito |
References:
Speaker: Camila Downey (Chile)
Embryological & Pathogenic Link:
Types and Severity of RASopathies:
| RASopathy Type | Extent of Cell Involvement | Examples | Notes |
|---|---|---|---|
| Germline (all cells affected) | Systemic | Noonan Sme, NF1 | Requires mild/moderate dysregulation to allow survival |
| Mosaic (tissue-specific) | One/few tissues | Shimmelpenning | Severity depends on mutation timing and affected cells |
Genetic Mutations & Dermatologic Lesions:
| Mutation | Associated Lesions | |
|---|---|---|
| K-RAS | Nevus sebaceous, capillary malformations, AVMs | |
| H-RAS | Keratinocytic epidermal nevi, Spitz nevi | |
| N-RAS | Congenital melanocytic nevi | |
| B-RAF | Spitz nevi, congenital melanocytic nevi | |
Clinical Syndromes (“True RASopathies”) with Mosaic Presentation:
Specific dermatologic and extracutaneous findings:
| Syndrome | Mutation | Key Features |
|---|---|---|
| Shimmelpenning (Linear Nevus Sebaceous Syndrome) | K-RAS | Linear nevus sebaceous, coloboma, PDA, cerebral malformations |
| Keratinocytic Epidermal Nevus Syndrome | Widespread keratinocytic nevi, lymphatic malformation, cardiac anomalies | |
| Oculoectodermal Syndrome | Aplasia cutis, eyelid tags, dermoids, neurodevelopmental delay, cardiac anomalies | |
| Encephalocraniocutaneous Lipomatosis | Alopecia, orbital tags, CNS lipomas, seizures, ocular defects | |
| Phacomatosis Pigmentokeratotica | H-RAS | Sebaceous + melanocytic nevi, scoliosis, rhabdomyosarcoma, epilepsy |
| Cutaneous Skeletal Hypophosphatemia Syndrome | Epidermal nevi, rickets, bone demineralization, eye/CNS involvement |
Genotype-Phenotype Correlations:
Clinical Work-Up Recommendations:
Evaluate for systemic involvement when:
Suggested assessments:
Research Insight:
Key Takeaways:
References:
Speaker: Irene Lara Corrales (Canada)
1. Congenital Melanocytic Nevus (CMN)
2. Tuberous Sclerosis Complex (TSC)
3. Neurofibromatosis Type 1 (NF1)
| Disorder | Genetic Pathway | Targeted Therapy | Outcome/Notes |
|---|---|---|---|
| CMN (NRAS/BRAF fusion) | MAPK | Trametinib | ↓ lesion bulk, pruritus, erythema |
| CMN (PIK3CA-related) | PI3K-AKT-mTOR | PIK3CA inhibitors | ↓ melanocyte density (early evidence) |
| Tuberous Sclerosis | mTOR | Everolimus / Sirolimus | ↓ renal tumors; variable angiofibroma response |
| EGFR | Afatinib + Everolimus | ↓ SEGA and cortical lesions (preclinical) | |
| – | Prenatal Sirolimus | ↓ fetal rhabdomyomas (case reports) | |
| Neurofibromatosis Type 1 | RAS-MAPK | Selumetinib | ↓ inoperable plexiforms, improved QoL |
| Trametinib (gliomas) | FDA-approved for brain tumors |
References:
Speaker: Maria Agustina Acosta (Uruguay)
General Role & Indications
Phototherapy Modalities
| Modality | Comments |
|---|---|
| Narrowband UVB (NB-UVB) | Most commonly used in children |
| PUVA (Psoralen + UVA) | Rare in pediatrics due to safety concerns |
| Targeted phototherapy | Used for localized lesions |
| Home-based UVB | An emerging alternative with careful supervision |
Pediatric Considerations
Home Phototherapy
Phototherapy vs Biologics
References:
Speaker: Matias Maskin (Argentina)
Phototherapy is now generally used as a second-line therapy or in combination with biologics for severe cases.
Phototherapy is recommended for moderate cases, but systemic biologics are preferred for severe atopic dermatitis
Phototherapy remains a viable option for early-stage cases
The role of phototherapy in pediatric dermatology is evolving. While it remains an essential treatment option for mild to moderate conditions, newer biologic therapies are increasingly becoming the go-to treatments for more severe conditions. Moving forward, phototherapy will likely be incorporated more into combination therapies, making it a valuable tool but no longer a first-line treatment in most cases.
References:
Speaker: Henry W. LIM (USA)
Composition and Effects of Sunlight
| Darker skin: |
Lighter skin:
|
| More and larger melanosomes, individually dispersed | Smaller melanosomes, grouped in keratinocytes |
| Intrinsic SPF ~13 | Intrinsic SPF ~3 |
Family & Cultural Influence
Clinical Correlations
Age-Specific Advice
Practical Challenges
Skin Tone–Specific Recommendations
| Skin Type | SPF Recommendation | UVA Protection | Visible Light Protection |
|---|---|---|---|
| Type I–III (Light) | ≥ SPF 50 | Moderate | Not critical |
| Type IV–VI (Dark) | ≥ SPF 30 | Higher preferred | Important (pigmentation risk) |
Sunscreen on exposed areas (SPF and spectrum tailored to skin type)
+
References:
Speaker: Antonio Torrelo (Spain)
Overview: Phototherapy is a valuable tool in dermatology, including pediatric cases, though protocols are often adult-centered. Its application in children must be individualized, especially in rare or severe cases. The following summarizes insights into photodermatoses such as Polymorphic Light Eruption (PMLE) and Actinic Prurigo (AP), with recent treatment updates.
Systemic antioxidants: Safe for children (e.g., beta-carotene), less standardized in children.
| Condition | Presentation in Children | First-line Therapy | Emerging/Alternative | Remarks |
|---|---|---|---|---|
| Polymorphic Light Eruption (PLE) | Rare, mild; juvenile spring eruption | UV-A protection, antioxidants | Omalizumab (adults), topical tacrolimus |
Desensitization rarely used in <7 years |
| Actinic Prurigo (AP) | Common in Latin America; rare in Europe | Steroids (low response), avoidance | Talidomide, JAK inhibitors, nicotinamide | Associated with specific HLA types |
| Photodermatoses (refractory) | Severe, resistant to topical therapy | Photoprotection, antioxidants | JAK inhibitors, nicotinamide | Requires individualized approach |
References:
Speaker: Fernando M. Stengel (Argentina)
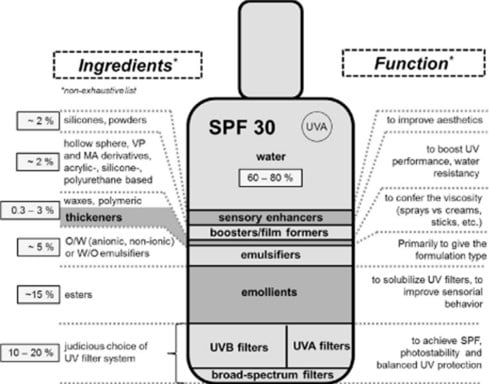
Pollution Sources
Coral Reefs and UV Filters
Geographic Evidence
| Compound | Category | Environmental Concern | Human Safety | Comment |
|---|---|---|---|---|
| Oxybenzone (BP-3) | Organic | Coral bleaching, endocrine disruption in fish | Potential endocrine disruptor | Banned in several regions |
| Octinoxate | Coral toxicity, marine pollution | Moderate absorption, hormonal data | Banned in reef-sensitive areas |
|
| Octocrylene | Marine toxicity, bioaccumulation | May accumulate in tissue | Still widely used in many countries | |
| Titanium Dioxide |
Inorganic (mineral) |
Precipitates in seawater, low coral interaction | Not absorbed dermally | Considered safe; used in children |
| Zinc Oxide | Similar to TiO₂ – precipitates, low coral impact | Not absorbed dermally | Recommended for pediatric use | |
| New Organic Agents | Organic hybrid | Limited data | Under investigation | Safer formulations under development |
References:
Report written by Dr Paola Stefano (Paediatric Dermatologist, Argentina)
Panellists: Dr Juan Carlos Lopez Gutierrez (Spain), Dr Eulalia Baselga (Spain), Dr Isabel Colmenero (Spain), Dr María Rosa Cordisco (United States)
In this session, Dr Maria Rosa Cordisco first announced the publication of the book ‘Anomalías vasculares en la infancia’, in Spanish, with the participation of international experts in genetics, radiology, dermatology, pharmacology and other disciplines. She explained the importance of interdisciplinary teamwork for the diagnosis, treatment and follow-up of these anomalies. Thanks to recent advances in genetics, classifying and diagnosing the different anomalies and establishing treatment with targeted therapies has been possible in these complex patients. Dr Natalia Torres emphasised that the book is up-to-date with the latest diagnostic and therapeutic advances, as well as the latest 2025 ISSVA classification.
In turn, Dr Teplinsky, a Paediatric Radiologist and Coordinator of the Vascular Anomalies Group at Hospital de Pediatría “Prof. Dr Juan P. Garrahan” [“Prof. Dr Juan P. Garrahan” Paediatric Hospital] and of the Centro de Anomalías vasculares del Sanatorio Mater Dei [Mater Dei Sanatorium Vascular Anomalies Centre], announced the creation of the Ibero-American Society for Vascular Anomalies (SIAV). This Society was founded in September 2024, is based in Spain and includes Spanish-speaking countries. This Society’s mission is to promote knowledge in the diagnosis and treatment of vascular anomalies and to foster collaboration among the different specialities and countries in Latin America, thereby contributing to improving the quality of life of patients. Another objective of this Society is to work together in the same language, in order to give correct nomenclature to vascular anomalies, create diagnosis and treatment guidelines and hold meetings to discuss cases. He also reinforced the desire for this new Society to be linked to the parent societies the Spanish Society of Vascular Anomalies (SEAV) and ISSVA (International Society for the Study of Vascular Anomalies).
Speaker: Dr Natalia Torres (Argentina)
Paediatric Dermatologist. Hospital Dr Juan P. Garrahan, Coordinator of the Vascular Anomalies Interdisciplinary Group - Hospital Dr Juan P. Garrahan - Argentina
The case of a 12-year-old girl was presented for discussion who had been seen for headache, pain and functional impotence of her arm. The patient also had macrocephaly and cognitive deficit. On dermatological examination, she had a vascular-looking, erythematous-telangiectatic lesion with evidence of venous pathways on her forearm from the first years of her life that progressed with her growth, and another lesion in her thoracic region. She had undergone two embolisations and was seeking a second opinion.
Ultrasound showed soft tissue enlargement and dilated vessels with high flow. Magnetic resonance angiography imaging also showed multiple arteriovenous shunts dependent on the subclavian, axillary and brachial arteries.
In addition, she had left ventricular dysfunction secondary to her vascular anomaly which generated volume overload, so she was administered enalapril and the decision was made to perform a new embolisation.
A skin biopsy was performed, confirming the diagnosis of an arteriovenous malformation.
She was administered thalidomide 50 mg/day and contraception was indicated. The patient continued to experience chronic pain and progressive loss of arm function.
The suspected diagnosis was PHOST syndrome (PTEN hamartoma of soft tissue) related to PTEN hamartoma tumour syndrome (PHTS), so a genetic study was requested and, in consultation with Dr Denise Adams, treatment with sirolimus was initiated. During the clinical course, the patient presented with bleeding ulcers and uncontrollable pain, so after an interdisciplinary evaluation, the decision was made to amputate her arm. The patient recovered her cardiac function. She continues to be treated with sirolimus for the chest lesion. In the end, the PTEN gene mutation was confirmed.
It is an underdiagnosed disease which requires early diagnosis to initiate appropriate treatment, and tumours can develop in adulthood.
Dr Juan Lopez Gutierrez suggested a registry of patients with PTEN mutations in order to identify different manifestations and associations in these patients.
Speaker: Dr Maria Laura Cossio (Chile)
Dr Cossio presented the case of a patient with the diagnosis of intracranial arteriovenous malformation with a large secondary cranial defect.
This was a newborn baby girl with congenital erythematous lesions on her scalp and convulsions at one month of age. MRI: right frontotemporal infarction with haemorrhages in the right parenchyma and cranial bone defect.
She initiated treatment with sirolimus as the scalp lesions started bleeding.
A biopsy of the scalp lesion could not be performed for genetic study, due to the associated risk of haemorrhage.
Dr Juan Carlos Lopez Gutierrez believed that MEK inhibitors such as trametinib could be indicated, biopsy of the scalp lesions could be performed if possible or perhaps a genetic blood study could be attempted.
Speaker: Dr Alejandro Celisv (Mexico)
A 29-year-old patient, who has had a bluish tumour on their lower lip since the age of 16 with gradual growth. At the age of 19, the patient presented with significant bleeding. The patient underwent embolisation with coil embolisation of nutrient vessels but continued to bleed and had to undergo vessel ligation. They were administered thalidomide for 1 year and remained stable. Surgery was then performed, following embolisation with resection of the tumour and reconstruction with skin flaps. The patient is currently on thalidomide and asymptomatic.
As comments, emphasis was placed on the early and combination treatment of arteriovenous malformations.
Speaker: Dr Irene Lara Corrales (Canada)
She presented a female patient with CM-AVM (capillary malformation associated with arteriovenous malformation).
This patient was seen at the age of 6 for asymmetric growth in a foot in which she had a capillary malformation (CM).
Genetic testing confirmed RASA1 mutation, both in skin biopsy and in blood. Genetic studies in both parents were negative.
An MRI of the brain and spine showed a cerebral arteriovenous (AV) fistula.
The CNS fistula was embolised, with no recurrence to date.
CM-AVM is an autosomal dominant inherited RASopathy.
Approximately 18% of patients with RAS 1 mutations have AV malformations or fistulas.
Dr Baselga suggested that newly diagnosed patients and adolescents should be asked to undergo follow-up brain and spine MRI scans prior to discharge, even if they are asymptomatic.
Speaker: Dr Felipe Enrique Velasquez Valderrama (Peru)
He presented a one-month-old patient with a congenital, vascular-looking, erythematous-violaceous tumour on the thigh, with a central crust.
The patient was hospitalised for systemic infection. Doppler ultrasound was performed on the tumour: thickened saphenous vein, collateral branches, tumour with venous and arterial components.
Days later the tumour started bleeding. All attempts were made to stop the bleeding, but the patient died from profuse haemorrhage.
Comments from Dr Colmenero: Rapidly involuting congenital haemangiomas (RICH) can have this fatal outcome. When prominent arterial vessels are detected on ultrasound, they should be embolised early.
Coordinator: Dr Agustina Vila Echague
Co-Coordinator: Dr Mirna Erendira Toledo Bahena & Dr Héctor Cáceres Ríos
Speaker: Betina Pagotto (Argentina)
Acne is a chronic inflammatory disorder of the pilosebaceous unit, which mainly affects adolescents and may continue into adulthood. It is estimated that it affects approximately 90% of the population at some point in their lives. Its main predisposing factors include age, skin type, obesity and family history.
Prevalence is almost 100% in adolescence and decreases with age. Its impact goes beyond the skin: it is associated with impaired quality of life, depression and low self-esteem, which highlights the importance of early treatment, especially in inflammatory cases.
The pathogenesis of acne is multifactorial:
Recently, the following new factors have been identified as being involved:
Different technologies have been used for the management of active acne and its sequelae:
Mechanisms of action
These therapies, especially when properly combined, can improve the inflammatory component, reduce bacterial colonisation and treat sequelae with remarkable aesthetic results.
Speaker: Dr Agustina Vila Echague (Salvador)
Capillary vascular malformations (CVMs or port-wine stains) are low-flow vascular malformations, present since birth, caused by genetic GNAQ mutation leading to progressive ectasia of the superficial cutaneous vascular plexus. They affect 0.3% of newborns.
Clinical presentation: They can present in different clinical subtypes: from flat, pale pink macules to violaceous, hypertrophic or nodular lesions. A significant percentage of these lesions are associated with Sturge-Weber syndrome, which accounts for approximately 20% of all vascular malformations. Symptoms of this syndrome may not be present at birth, but manifest progressively up to the age of 5. For this reason, performing clinical and neurological examinations is key for at least the first five years of life in patients with facial port-wine stains to confirm or rule out the presence of the syndrome.
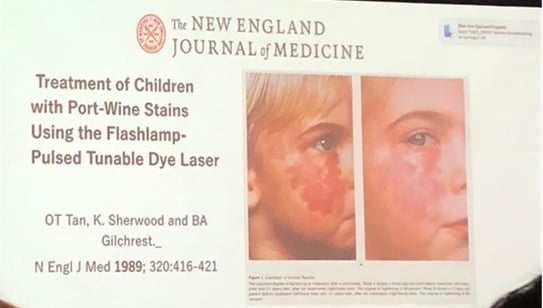
CVMs can be treated with pulsed-dye laser (PDL, 595 nm). Initiating treatments at an early age is recommended, as vessels are more superficial and smaller.
Reference: He HY, Shi WK, Jiang JC, Gao Y, Xue XM. An exploration of optimal time and safety of 595-nm pulsed dye laser for the treatment of early superficial infantile hemangioma. Dermatol Ther. 2022 May;35(5):e15406. doi: 10.1111/dth.15406. Epub 2022 Mar 9. PMID: 35199898; PMCID: PMC9285537.
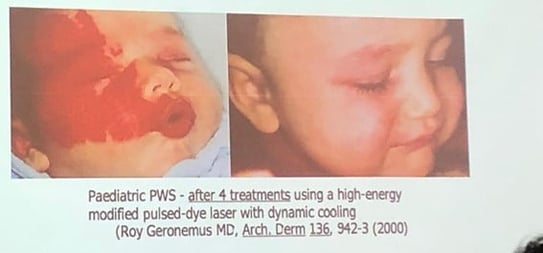
Reference: Geronemus R et al. High fluence modified pulsed dye laser photocoagulation with dynamic cooling of port wine stains in infancy. Arch Derm 2000; 136: 942–943
Speaker: Héctor Cáceres Ríos (Peru)
He initially referred to infantile haemangiomas, benign tumours with endothelial proliferation. They usually onset in the first few weeks of life and grow rapidly for the first 3 months. They then remain stable in growth or grow very little until one year of age and then begin to involute and slowly decrease in size. There are precursor signs in up to 50% of cases: telangiectasias, anaemic macules or bluish spots.
There are small lesions, with no clinical repercussions, and others that are located near vital organs such as the eyes and mouth, for which treatment must be defined.
Clinically, they may be superficial in 60% of cases (“strawberry” variant), deep (bluish in colour) in 15%, or mixed in 25%.
Differentiating them from neoplastic processes such as lymphomas or rhabdomyosarcomas is essential.
As for their origin, they have been found to share the same markers as the placenta, which backs the theory of placental tissue embolisation. GLUT-1, merosin, FcYRII and Lewis Y antigen expression, among others, have been detected, confirming that haemangiomas represent vasculogenesis and angiogenesis processes, influenced by different factors.
Combination therapy: he also mentioned the possibility of combining topical and systemic laser treatments.
Current knowledge about the genetics of vascular anomalies and the availability of effective laser technologies enables a more precise and personalised approach. Early initiation of treatment improves clinical outcomes and quality of life for paediatric patients.
References:
Asilian, Ali1,2,3; Mokhtari, Fatemeh1,3; Kamali, Atefeh Sadat1,3,4,; Abtahi-Naeini, Bahareh1,3; Nilforoushzadeh, Mohammad Ali2,5; Mostafaie, Shayan3,6. Pulsed dye laser and topical timolol gel versus pulse dye laser in treatment of infantile hemangioma: A double-blind randomized controlled trial. Advanced Biomedical Research 4(1):p 257, | DOI: 10.4103/2277-9175.170682
Kavitha K. Reddy MD, Francine Blei MD, Jeremy A. Brauer MD, Milton Waner MD, Robert Anolik MD, Leonard Bernstein MD, Lori Brightman MD, Elizabeth Hale MD, Julie Karen MD, Elliot Weiss MD, Roy G. Geronemus MD Retrospective Study of the Treatment of Infantile Hemangiomas Using a Combination of Propranolol and Pulsed Dye Laser. Dermatol Surg Volume39, Issue6. June 2013.Pages 923-933.https://doi.org/10.1111/dsu.12158
Speaker: Dr Mirna Erendira Toledo Bahena (Mexico)
Dr Toledo Bahena first referred to laser advances over the last 30 years that have led to the development of new therapeutic strategies for common paediatric skin conditions. She emphasised laser treatment as a non-invasive alternative to surgical options, and in many cases, it can provide better results.
In expert hands, laser therapy has been shown to be safe in both children and adults. This is very important and receiving specific training in treating paediatric patients is essential.
In the paediatric population, the scope of treatment has been extended to a variety of conditions including vascular and pigmented lesions, inflammatory diseases, scars, vitiligo and for tattoo and hair removal as well.
The laser works by emitting high-intensity monochromatic light, which is absorbed by specific structures in the skin called chromophores. This absorption generates selective thermal destruction of the target without damaging the surrounding tissue. In vascular lesions, the chromophore is haemoglobin, and in pigmented lesions, it is melanin.
In pigmented lesions, the chromophore is melanin; in vascular lesions, it is haemoglobin. Light absorption varies from 250 to 1200 nm, depending on lesion depth.
Q-switched or picosecond lasers fragment the pigment so that it is eliminated by the lymphatic system. These are ideal for many of the conditions we treat in paediatric dermatology, including tattoos.
In pigmented lesions such as naevus of Ota, the gold standard is the Q-switched laser. Nd:YAG, Ruby or Alexandrite lasers are used. These act via photoacoustics, destroying the dermal pigment.
One study reported that approximately 5 Alexandrite laser sessions achieved 50% effectiveness with a low adverse event rate (between 2% and 7.9%). Dr Toledo Bahena reported that, at her hospital, they have treated 15 patients (aged 3–16 years, phototype IV) and assessed their response after several Q-switched Nd:YAG sessions.
They obtained:
• 45% with excellent response
• 13% with moderate response
• 25% with poor response
Only one patient showed residual hyperpigmentation.
The doctor showed several photographs of children treated for naevus of Ota who showed good results.
She also spoke about the excimer laser for pigmentary conditions:
Although it is not strictly a laser, excimer light (308 nm) is a very useful technology for diseases such as vitiligo. It works by inducing apoptosis in lymphocytes and keratinocytes, and stimulates melanocyte migration and melanin production.
It is a safe option that only acts on the epidermis, which is ideal for children and patients with sensitive skin.
References:
Sakio R, Ohshiro T, Sasaki K, Ohshiro T. Usefulness of picosecond pulse alexandrite laser treatment for nevus of Ota. Laser Ther. 2018 Dec 31;27(4):251–255. doi:10.5978/islsm.27_18-OR-22
Mejoría clínica de pacientes pediátricos con nevo de ota utilizando tecnología láser Q-Switched en el Hospital Infantil de México Federico Gómez. https://hdl.handle.net/20.500.14330/TES01000761986
Speaker: Jean L. Bolognia (United States)
Dr Jean L. Bolognia proposed a clinical and pathological journey along the “blue brick road”, from congenital sacral dermal melanocytosis to cellular blue naevi, with emphasis on the clinical, histological, genetic aspects and diagnostic implications of these pigmented melanocytic lesions. Entities such as acquired and congenital forms of the naevus of Ota and Ito, as well as the association with neurocutaneous syndromes, malignant potential and therapeutic management were discussed.
1. Congenital dermal melanocytosis (CDM)
2. Naevus of Ota and Ito
Naevus of Ota: also called oculodermal melanocytosis. It involves V1-V2 dermatomes, is usually unilateral and has ocular involvement (50% in mild forms, up to 100% in extensive forms).
Naevus of Ito: similar, but in brachial nerve territories; no ocular involvement.
In addition, she described acquired bilateral naevus of Ota-like macules (ABNOM), also known as Hori’s naevus, a benign dermal melanocytosis characterised by the appearance of blue-brown or slate grey macules on the face, especially on the cheeks, temples and forehead. It is most commonly seen in women of Asian descent after the third decade of life. ABNOM differs from naevus of Ota in that its onset occurs later and there is no involvement of the conjunctiva, mucosa and tympanic membrane.
3. Common genetic aspects
4. Blue naevi
“Common” and “cellular” are histological terms, not clinical ones.
Combined: association of compound naevus with blue naevus (greyish colour).
Histology is key to differentiating benign from malignant forms.
5. Variants and related entities
Congenital dermal melanocytosis and the different types of blue naevi represent a broad spectrum of melanocytic lesions with diverse clinical, genetic and prognostic implications. Detailed knowledge of its clinical presentation, anatomical distribution, histological characteristics and syndromic associations enables an accurate diagnosis to be made, cases at risk of malignancy to be identified, and appropriate follow-up to be implemented, including ophthalmological monitoring and possible CNS imaging in selected patients. Identifying GNAQ/GNA11 mutations and BAP1 loss are key in understanding its biology and potential for malignant transformation.
References:
Cordova A. The Mongolian spot: a study of ethnic differences and a literature review. Clinical Pediatrics 1981 vol 20: 714–719
Romagnuolo M, Moltrasio C, Gasperini S, Marzano AV, Cambiaghi S. Extensive and Persistent Dermal Melanocytosis in a Male Carrier of Mucopolysaccharidosis Type IIIC (Sanfilippo Syndrome): A Case Report. Children (Basel). 2023 Dec 13;10(12):1920. doi: 10.3390/children10121920. PMID: 38136122; PMCID: PMC10742075.
Speaker: Dr Amy Paller (United States)
Pruritus is a common manifestation in a variety of dermatoses, but its treatment in genetic diseases such as ichthyosis (now called epidermal differentiation disorders) and epidermolysis bullosa (EB) represents a considerable clinical challenge.
Up to 17% of children and adolescents report chronic pruritus, and it is even more prevalent in genetic skin diseases. Scales such as the NRS (Numerical Rating Scale) or the VAS (Visual Analogue Scale) have been validated to quantify intensity, including versions adapted for parents of children who cannot self-assess. However, it highlights the need for more holistic assessments that take into account the impact on physical, mental and social health.
Devices such as sensorised gloves, digital sensors and portable acoustic systems (e.g. ADAM sensor) have been developed that are able to record scratching events during sleep, differentiating these from other movements.
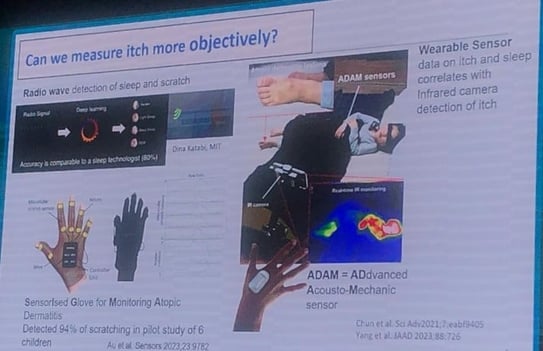
Pruritus is transmitted by C- and A-delta nerve fibres, with endings reaching the epidermis. These neurons express key receptors such as IL-4, IL-13 and IL-31 and communicate bidirectionally with keratinocytes and immunocytes, amplifying or regulating the pruritic stimulus.
Methicillin-resistant Staphylococcus aureus (MRSA) induces pruritus in murine models by producing V8 protease, which activates the PAR-1 receptor on nerve fibres. This pathway, which is independent of histamine and ILs, opens up novel therapeutic opportunities with topical PAR-1 inhibitors.
Up to 93% of patients present with pruritus. In studies with pregabalin, a benefit on neuropathic pain was found, but with little effect on pruritus. In murine models, the use of endocannabinoid system modulators has been explored, showing promising results.
Increased substance P has been documented in the skin of patients with dystrophic EB, correlating with increased pruritus intensity. Neurokinin 1 (NK-1) receptor antagonists such as serlopitant have shown a tendency to reduce pruritus in pilot studies.
A recent survey reported that 45% of patients with EB use tetrahydrocannabinol/cannabidiol (THC/CBD) products, and 64% report improvement of pruritus. Cannabinoid CB1 and CB2 receptors are present in dorsal root ganglia and skin fibres, and alterations in their expression have been identified in animal models.
Current therapies: biologics and JAK inhibitors
This biologic, fully human monoclonal antibody (mAb) targets the α-subunit of the interleukin 4 receptor (anti-IL-4Rα) and is approved for atopic dermatitis. It has been evaluated in an open-label study in patients with epidermolysis bullosa (n=22). At 16 weeks, a significant reduction in mean and severe pruritus was found, as were objective improvements in sleep using recording devices. Response was independent of baseline IgE levels.
In a subgroup with junctional epidermolysis bullosa (collagen VII deficiency), a reduction of more than 4 points on the pruritus scale was documented, with a positive impact on autonomy and quality of life. Safety was favourable, with minimal local reactions and only one case of mild conjunctivitis.
IL-31R antibody (nemolizumab): with therapeutic potential, especially because of the correlation of IL-31 with pruritus in EB.
JAK inhibitors: used off-label (baricitinib, tofacitinib, etc.) with good results in reducing pruritus and lesions in EB, although their risk profile in immunocompromised patients is of concern.
Pruritus is multifactorial in genetic diseases, with complex neuroimmunological mechanisms. The use of objective tools, together with targeted therapies (biologics, cannabinoids, specific pathway inhibitors), opens a new paradigm in the personalised treatment of these patients. Further studies are needed to optimise safe and effective therapeutic strategies in the affected paediatric population.
Reference:
Deng L, Costa F, Blake KJ, Choi S, Chandrabalan A, Yousuf MS, Shiers S, Dubreuil D, Vega-Mendoza D, Rolland C, Deraison C, Voisin T, Bagood MD, Wesemann L, Frey AM, Palumbo JS, Wainger BJ, Gallo RL, Leyva-Castillo JM, Vergnolle N, Price TJ, Ramachandran R, Horswill AR, Chiu IM. S. aureus drives itch and scratch-induced skin damage through a V8 protease-PAR1 axis. Cell. 2023 Nov 22;186(24):5375-5393.e25. doi: 10.1016/j.cell.2023.10.019. PMID: 37995657; PMCID: PMC10669764.
Speaker: Dr Giovanni Pellacani (Italy)
This session addressed the most relevant criteria for the differential diagnosis of melanocytic naevi, the importance of clinical follow-up and the indications for excision. He referred mainly to melanoma, an extremely rare malignancy in the paediatric population. Its rarity, coupled with the extensive clinical and dermoscopic variability of naevi in children, poses significant diagnostic and therapeutic challenges.
Useful for identifying patterns suggestive of malignancy such as:
At school age, lesions may appear with:
Lesions with:
In these cases, diagnostic excision with adequate margins is recommended.
Case report presented:
Melanoma in childhood is rare but clinically significant.
Any medium or large congenital naevus should be carefully evaluated and monitored over time.
Physical examination and dermoscopy are essential for decision-making.
Excision with adequate margins should be indicated for any suspected clinical or dermoscopic atypia.
It is essential to avoid both under- and overestimation of pigmented lesions in children.
Reference:
Excised melanocityc lesions in children and adolescents-a 10 year survey. E Moscarella 1, I Zalaudek, L Cerroni, I Sperduti, C Catricalà, J Smolle, R Hofmann-Hellenhof, A Sgambato, G Pellacani, G Argenziano. Br J Dermatol. 2012 Aug;167(2):368-73. doi: 10.1111/j.1365-2133.2012. 10952.x
Speaker: Dr Horacio Cabo (Argentina)
Nail pigmentation is a common reason for dermatology consultations, and although most of the time these are benign lesions, there is a small but significant risk of subungual melanoma, even in children. This talk addressed diagnostic difficulty, dermoscopy use and key clinical criteria to differentiate between benign melanonychia and melanoma.
Pathophysiology and clinical presentation
Subungual melanoma: epidemiology and case reports
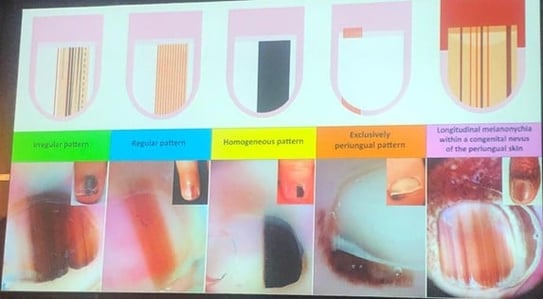
Speaker: Dr Virginia Gonzalez (Argentina)
Dermoscopy is no longer a tool exclusively for evaluating pigmented lesions, but has become an essential method for the diagnosis of various inflammatory, infectious and parasitic dermatoses. It has been called “the dermatologist’s stethoscope”, and its systematic use after physical examination improves diagnostic accuracy, avoids unnecessary biopsies and facilitates therapeutic follow-up.
Fundamentals of dermoscopic analysis in inflammatory diseases
Highlighted clinical examples
Psoriasis
Eczema
Pityriasis rosea
Porokeratosis
Granulomatous diseases (e.g. sarcoidosis, granulomatous rosacea)
Lichens and pruritic diseases
Scabies
Pediculosis (lice)
Molluscum contagiosum
Warts (HPV)
Colouring and fluorescence: emerging keys
Dermoscopy is an invaluable diagnostic tool in general dermatology, and it should be used beyond pigmented lesions.
The use of UV light enables detecting fluorescent findings useful in several inflammatory and infectious pathologies.
Its appropriate use can avoid unnecessary biopsies, improve therapeutic monitoring and increase clinical precision.
Using dermoscopy in the routine evaluation of dermatoses and becoming familiar with the characteristic signs of each condition is recommended.
Speaker: Dr Rosario Peralta (Argentina)
Dermoscopy is an essential tool in the non-invasive assessment of vascular skin lesions. In paediatrics, it enables better characterisation of tumours and vascular malformations, optimising the differential diagnosis in the presence of melanocytic or even malignant lesions. Recognising specific vascular patterns facilitates classifying and treating these lesions, often without the need for biopsy.
1. Infantile haemangioma
- Most common benign vascular lesion in children
- Well-defined reddish-violet lacunae of different sizes
- Homogeneous or mosaic pattern; no additional structures
- Clinical course: colours vary according to the depth of the vascular component
2. Tufter angioma (“tufted haemangioma”)
- Prominent peripheral linear vessels, often accompanied by areas of erythrosis
- Frequent structural variability: structurally specific white areas can be seen
3. Glomangioma
- Painful, bluish or blackish lesion
- Dermoscopy: blue-violet areas, white-blue veil structure and occasional rainbow pattern
- Signs of thrombosis: homogeneous black areas or “black lagoon” structures
4. Angiokeratoma
- Red, dark blue or black lacunae, sometimes with hyperkeratosis
- Presence of whitish areas and frequent thrombosis
- Differential diagnosis with metastatic melanoma or nodular melanoma
5. Lymphangioma (superficial lymphatic malformation)
- Yellowish-whitish or pinkish lacunae, sometimes mixed with reddish-violet lacunae
- Polymorphic pattern: mix of clear and haemorrhagic lacunae
- Under UV dermoscopy: fluorescence varies according to liquid content
6. Angiosarcoma (take into account in differential diagnosis)
- Although rare in children, it should be considered for rapidly growing vascular tumours
- Dermoscopy: homogeneous reddish areas with irregular vascular lines and ulceration areas
- Important to suspect it with atypical lesions that mimic haemangiomas
7. Capillary and venous malformations
- Capillary malformation: red, linear or serpiginous spots, depending on whether they are superficial or deep
- Venous malformation: bluish or purplish, often compressible lacunae, without specific structures
- Distribution according to vessel location (horizontal or vertical in superficial or deep dermis)
Properly interpreting these findings, coupled with clinical impression, prevents unnecessary procedures such as biopsies in children and improves the therapeutic approach.
Speaker: Dr Ramiro Cano (Argentina)
Trichoscopy is a non-invasive tool that has revolutionised the diagnostic approach to childhood alopecia. It enables assessing the scalp and hair shafts via dermoscopy, facilitating the differential diagnosis between the different aetiologies of alopecia. This talk focused on the clinical applications of trichoscopy to differentiate the most common causes of patchy alopecia in children, mainly tinea capitis, alopecia areata and trichotillomania.
1. Three-step diagnostic approach
Step 1: Medical history and detailed physical examination to classify the alopecia as:
Step 2: Initial trichoscopic assessment to define if it is:
Step 3: Detailed trichoscopy to identify specific patterns and confirm the presumptive clinical diagnosis.
2. Classification and trichoscopic patterns
Trichotillomania
Note: Most of the patterns are non-specific, except for some that have a higher positive predictive value
Fibrous (irregular): indicative of cicatricial alopecia
3. Trichoscopic findings most useful in differential diagnosis
|
Corkscrew, zigzag, block, Morse code-type hairs |
|
Exclamation mark hairs, vellus hairs, circle hairs, constrictions |
|
Hair dust, V-sign, flame hairs, occasional haemorrhages |


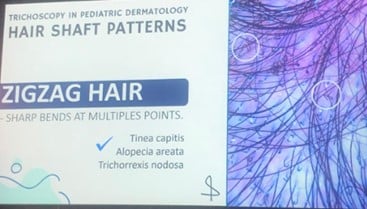
Trichoscopy is a useful and accessible technique for the diagnosis and follow-up of childhood alopecia, especially in the outpatient setting. Although many findings are shared by different conditions, some patterns have a high specific diagnostic value. A structured approach, based on medical history, physical examination and targeted trichoscopy, enables:
It is essential for paediatric dermatologists to become familiar with the key trichoscopic patterns to optimise the treatment of these conditions.
Speaker: Dr Gabriel Salerni (Argentina)
The speaker presented a selection of case reports observed in the paediatric population, with the aim of highlighting relevant clinical and dermoscopic patterns in the differential diagnosis of nodular and pigmented lesions. The topics ranged from benign and self-limiting entities such as idiopathic facial aseptic granuloma, to more serious pathologies such as childhood melanoma.
Patient: 9-year-old girl with a 3-week history of a 2 cm erythematous nodule on her cheek, with no history of trauma or sting.
Reference:
Errichetti E et al. Dermoscopy of idiopathic facial aseptic granuloma (IFAG): an observational controlled study Int Dermatol 2025 Feb; 64(2):422-424
Cases in children and adolescents, typically as red or pigmented nodules on the face or extremities.
Reference:
Lallas A, Apalla Z, Papageorgiou C, Evangelou G, Ioannides D, Argenziano G. Management of Flat Pigmented Spitz and Reed Nevi in Children. JAMA Dermatol. 2018 Nov 1;154(11):1353-1354. doi: 10.1001/jamadermatol.2018.3013.
Kerner M, Jaimes N, Scope A, Marghoob AA. Spitz nevi: a bridge between dermoscopic morphology and histopathology. Dermatol Clin. 2013 Apr;31(2):327-35. doi: 10.1016/j.det.2012.12.009. Epub 2013 Jan 30. PMID: 23557659.
16-year-old adolescent with a bleeding violaceous lesion on the knee
Reference
Zaballos P, Daufí C, Puig S, Argenziano G, Moreno-Ramírez D, Cabo H, Marghoob AA, Llambrich A, Zalaudek I, Malvehy J. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007 Mar;143(3):318-25. doi: 10.1001/archderm.143.3.318. PMID: 17372096.
Unusual case in a 12-year-old boy with a 3-month history of polymorphic leg lesion.
The classification was reviewed:
The use of the modified ABCD was discussed:
D: De novo
Two cases in 4-year-old children
Speakers: Dr Bruno Ferrari (Argentina), Dr Carla Torres Zegarra (United States)
- Molluscum contagiosum (MC) is a common childhood viral infection caused by a poxvirus that affects the skin and occasionally the mucosa.
- It presents as umbilicated papules which are usually asymptomatic and are located anywhere on the body.
- It is a self-limiting and benign disease in immunocompetent patients.
- CM usually resolves spontaneously within 6 to 12 months, although it can last up to 18 or even 30 months.
- During its course, local inflammation with signs of eczema (“beginning of the end” phenomenon) may develop, which usually precedes lesion resolution.
- The “scars” they usually leave are not permanent.
- In most cases no active treatment is required.
- Available therapies (e.g. cryotherapy, imiquimod, curettage, intralesional injections) can be painful, cause anxiety and scarring and require multiple medical visits.
- There is no conclusive evidence that treatment significantly accelerates resolution compared to watchful waiting.
- Cochrane Review 2017: no clear superiority found between treatments, nor against watchful waiting.
- Studies highlight that many treatments have limited efficacy (30–40%), and are associated with recurrences.
- Painful procedures generate anxiety in children and an aversion to future medical visits
- Multiple treatment sessions place an emotional and financial burden on families
- CM is mildly contagious, with an estimated intrafamilial transmission rate of 30–40%.
- Restricting children from their activities (e.g. school, swimming) is not recommended
- Basic hygiene measures are advised: avoid scratching, cover inflamed lesions, do not share personal hygiene items
- Immunocompromised patients
- Extensive or recalcitrant infections
- Facial or genital involvement with aesthetic or functional impact
- Lesions with severe secondary eczema
- Molluscum contagiosum is a self-limiting viral infection in most healthy children.
- Active treatment is not always necessary and risks may outweigh benefits.
- Watchful waiting should be considered as the first line of treatment in healthy patients.
- Informing parents and carers about the natural course of the disease and avoiding unnecessary treatment are essential.
Reference: van der Wouden JC, van der Sande R, Kruithof EJ, Sollie A, van Suijlekom-Smit LW, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017 May 17;5(5):CD004767. doi: 10.1002/14651858.CD004767.pub4. PMID: 28513067; PMCID: PMC6481355.
Speaker: Dr Maria Gnarra Buethe (United States)
The focus was on pain management, anxiety and the overall paediatric patient experience during minor dermatological procedures:
Distress during procedures in paediatric dermatology represents a major clinical challenge, both for patients and their families. This talk presented a multidisciplinary approach to reduce pain, anxiety and improve children’s experience through pre-, trans- and post-procedure interventions.
1. Pre-procedure preparation:
Initiating patient and carer orientation prior to the day of the procedure is recommended. Including a Child Life specialist, if available, improves understanding and reduces anxiety. The process can also be carried out in the procedure room, while consents are being reviewed.
2. Surgical setting organisation:
Preparing the material out of children’s field of vision and having everything ready for an efficient procedure (<30 seconds for punch biopsies) are key to reducing distress.
3. Patient positioning:
Different techniques of comfortable immobilisation, adapted to the age of the patient, were described:
Infants: Burrito wrap with sheets
Older children: Ergonomic positions on the carer’s lap, allowing access to different anatomical areas as needed
4. Distraction techniques:
Active distraction (e.g. video games) has been shown to be more effective than mild sedation (midazolam) and passive distraction (e.g. cartoons) in reducing both pain and anxiety. Prior familiarisation with the applications and their suitability for the procedure site (hands free if limbs are involved) is recommended.
5. Additional distraction tools:
Sensory toys and virtual reality (VR) were mentioned. Although VR and noise-cancelling headphones offer similar benefits, both have shown efficacy in reducing perioperative anxiety.
6. Validation and positive reinforcement:
Language should focus on reinforcing the child’s courage and avoiding negative associations (“it was painful”). Emphasis was placed on the use of distractor questions and emotionally validating statements.
7. Pain management:
Newborns: Skin-to-skin contact, breastfeeding and sucrose solution decrease signs of pain (facial grimace, heart rate).
Topical anaesthetics:
Caution with methaemoglobinemia in infants
Topical anaesthesia enhancement: Greater efficacy has been documented if applied after cryotherapy, due to the change in the epidermal barrier.
8. Infiltration anaesthesia:
Use small needles.
Warm lidocaine is less painful than cold lidocaine.
Buffered lidocaine (with sodium bicarbonate) decreases infiltration pain with no clear evidence of loss of potency or stability.
Injection technique: start in the deep dermis and advance superficially.
9. Complementary methods:
Local cooling: ice, ethyl chloride
Vibration (gating theory): the device should be placed proximal to the surgical site and between the surgical site and the brain.
Playful cryotherapy on the scalp: use of frozen water balloons for analgesia prior to infiltration
10. Sedation and general anaesthesia:
Nitrous oxide: commonly used in paediatric dentistry, effective as mild sedation, with need for monitoring for risk of hypoxaemia
General anaesthesia: parents should be informed about the FDA warning in children under 3 years of age for prolonged (>3 h) or repetitive procedures, such as laser treatments for capillary malformations.

Encourage a relaxed and calm environment to relieve the patient’s anxiety.
Apply topical anaesthetics whenever appropriate to reduce discomfort.
Inject anaesthetic slowly, starting at deeper levels for better tolerance.
Using distraction techniques, mobile phone apps, music, conversation or interaction with the carer can be effective.
Speaker: John Mc Grath (United Kingdom)
Genodermatoses represent a large group of inherited skin diseases, many of which have high morbidity and require complex diagnostic and therapeutic approaches. More than 8,000 Mendelian diseases are currently recognised, with more than 1,000 presenting with cutaneous manifestations. This talk provided a critical update on advances in molecular diagnostics and emerging therapeutic strategies, particularly focusing on epidermolysis bullosa (EB) and related conditions.
Example: Cobalamin J disease: autosomal recessive disease of vitamin B12 metabolism leading to progressive hyperpigmentation, which can be successfully treated with vitamin B12. The diagnosis was made with NGS which identified the ABCD4 gene. These untreated patients are at increased risk of stroke starting in their 20s.
Reference: Takeichi T, Hsu CK, Yang HS, Chen HY, Wong TW, Tsai WL, Chao SC, Lee JY, Akiyama M, Simpson MA, McGrath JA. Progressive hyperpigmentation in a Taiwanese child due to an inborn error of vitamin B12 metabolism (cblJ). Br J Dermatol. 2015 Apr;172(4):1111-5. doi: 10.1111/bjd.13413. Epub 2015 Feb 27. PMID: 25234635.
He also referred to the therapeutic complexity of epidermolysis bullosa due to its aetiopathogenesis. Dystrophic EB has a mutation in the type VII collagen gene with deficient formation of anchoring fibrils. In addition to structural damage, there is a significant systemic inflammatory involvement, driven by TGF-β, which promotes fibrosis and squamous carcinoma.
The approach to genodermatoses, and especially to EB, has shifted from a purely symptomatic approach to an era of precision medicine and targeted therapies. Molecular identification of genetic mutations has been key to establishing targeted therapies, some of them curative. However, accessibility, cost, and customisation of treatment are still major barriers. A holistic approach is required, combining primary treatment, inflammatory control and symptomatic care, all focused on the patient’s priorities.
Speaker: Dr Cristina Has (Germany)
Pharmacogenetics (PGx) and pharmacogenomics (PG) are rapidly evolving disciplines that study how individual genetic variability influences drug response. While pharmacogenetics focuses on the influence of individual genes, pharmacogenomics considers several genetic interactions. These areas have enabled progress towards personalised medicine, with greater therapeutic efficacy and lower risk of adverse effects, including severe cutaneous reactions (SCARs) such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).
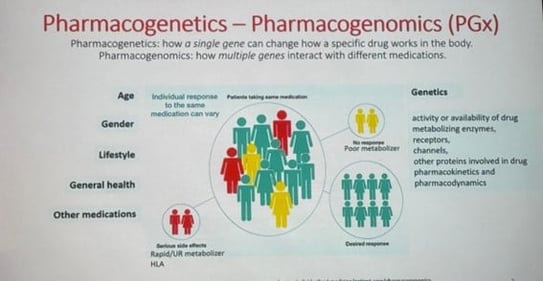
Reference: Kabbani et al. Pharmacogenomics in Practice: A Review and Implementation Guide. Frontiers in Pharmacology, 18 de mayo de 2023. DOI: 10.3389/fphar.2023.1189976
History and evolution
The term “pharmacogenetics” was coined in 1959.
Milestones: discovery of cytochrome P450 enzymes that control the metabolism of most drugs and HLA genes associated with hypersensitivity reactions.
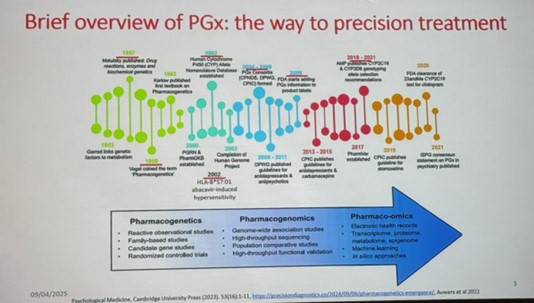
Major events in the emergence of pharmacogenomics in psychiatry. AMP, Association of Molecular Pathology; CPIC, Clinical Pharmacogenetics Implementation Consortium; CPNDS, Canadian Pharmacogenomics Network for Drug Safety; DPWG, Dutch Pharmacogenetics Working Group; FDA, Food and Drug Administration; ISPG, International Society of Psychiatric Genetics; PGRN, Pharmacogenomics Global Research Network; PGx, pharmacogenomic; PharmGKB, Pharmacogenomics Knowledge Base; PharmVar, Pharmacogene Variation Consortium.
Studies: observational, familial, candidate genes, clinical trials
Modern techniques: genome-wide association study (GWAS), high throughput sequencing (HTS), multi-omics technologies (genomics, transcriptomics, proteomics, metabolomics, etc.), artificial intelligence.
Requires: basic research → validation → databases →clinical guidelines → implementation.
International consortia such as CPIC® (Clinical Pharmacogenetics Implementation Consortium) or PharmGKB® generate evidence-based guidelines (for example) that are available to all.
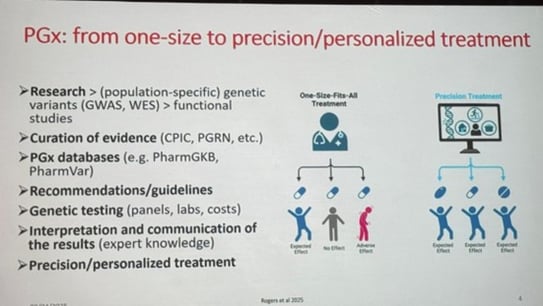

Reference: Auwerx, Chiara et al.From pharmacogenetics to pharmaco-omics: Milestones and future directions. Human Genetics and Genomics Advances, Volume 3, Issue 2, 100100
Point-of-care model: a gene or several genes and specific drugs are tested before treatment or when the patient does not respond to treatment or has experienced adverse reactions
Pre-emptive: preventive model where a broad genetic profile is studied, regardless of the patient’s medical history
Methods: candidate gene panels, exome or whole genome
Real-time customised prescription requires a complex workflow: informed consent, extraction, interpretation and treatment adjustment
Specialists needed: pharmacogeneticists and multidisciplinary teams
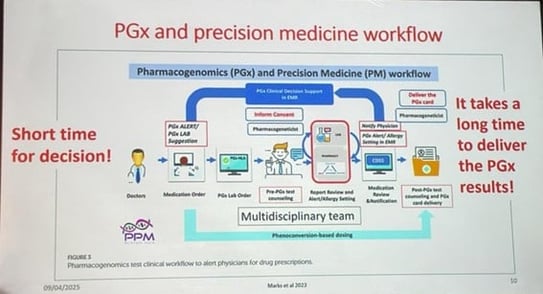
Reference:
Marks et al. Updates in SJS/TEN: collaboration, innovation, and community. Front. Med., 10 October 2023.Sec. Dermatology.Volume 10 2023 https://doi.org/10.3389/fmed.2023.1213889
Lack of generalised medical knowledge
Costs, sustainability, integration with clinical systems
Population variability in risk alleles
Drugs with genetic implications
e.g. Azathioprine: inherited thiopurine methyltransferase (TPMT) deficiency can lead to severe toxicity; genetic testing is useful, but does not detect all cases → close clinical monitoring is required.
Severe cutaneous adverse reactions (SCARs)
Examples: SJS/TEN
High mortality (up to 50% in adults)
Associated with >80% with drugs such as: Allopurinol, aromatic anticonvulsants, sulphonamide antibiotics, NSAIDs, antiretrovirals
Pathophysiological mechanism
Type IV immune reaction mediated by cytotoxic T lymphocytes.
Risk factors: HLA alleles, impaired drug metabolism (e.g. CYP2C9), viral infections, drug-drug interactions
Population variability
HLA risk alleles vary according to ethnicity (e.g. HLA-B*15:02 in Asians for carbamazepine, HLA-B*57:01 in Europeans for abacavir).
No universal approach → requires local adaptation
Case reports and studies
Example of a patient with major depression treated unsuccessfully with multiple drugs → improvement after PGx test
Paediatric studies (Toronto): up to 80% with actionable variants after genomic sequencing
Actual implementation
Successful trials in psychiatry, oncology, cardiology
Reimbursement in some European countries, but still limited clinical use
Pharmacogenetics enables more effective and safer personalised medicine.
Effective clinical implementation requires continuing medical education, technological resources, scientific validation and adaptation to population genetic variability.
In dermatology, SCARs represent a critical application with a high clinical/prophylactic impact.
The future of personalised medicine includes preventive tests integrated into electronic medical records, with personalised risk lists for each patient.
References:
Cohn I, Manshaei R, Liston E, et al. Assessment of the Implementation of Pharmacogenomic Testing in a Pediatric Tertiary Care Setting. JAMA Network Open, 2021;4(5):e2110446
DOI: 10.1001/jamanetworkopen.2021.10446Europe PMC+3
Barker, C.I.S., Groeneweg, G., Maitland-van der Zee, A.H., Rieder, M.J., Hawcutt, D.B., Hubbard, T.J., Swen, J.J., & Carleton, B.C. (2021). Pharmacogenomic testing in paediatrics: Clinical implementation strategies. British Journal of Clinical Pharmacology, 88(10), 4297–4310
Speaker: Dr Maria Cecilia Rivitti Machado (Brazil)
Hidradenitis suppurativa (HS) is a chronic, recurrent inflammatory disease of the hair follicle, not of the sweat glands as previously thought. It typically presents with nodules, abscesses and tunnels (fistulous tracts), mainly in intertriginous areas. Despite its significant clinical and psychosocial burden, it continues to be underdiagnosed. This is especially true in the paediatric and adolescent population, where its incidence seems to be increasing.
Chronic, progressive inflammatory disease of the hair follicle
It is characterised by the presence of painful inflammatory nodules, abscesses and tunnels in typical locations such as the armpits, groin, buttocks and breast region.
Lesions recur ≥2–3 times in a six-month period.
Double comedones and nodules in both armpits :
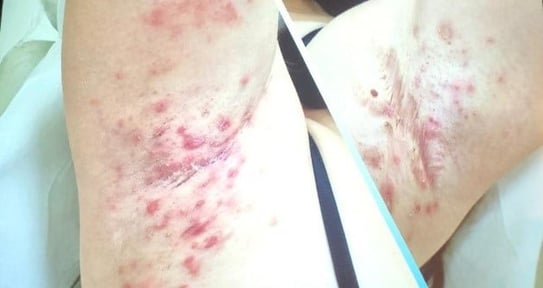
Retractable scars and armits tunnels :
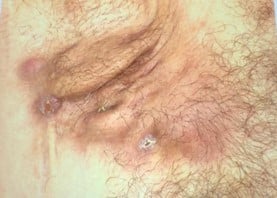
It is not a rare disease, although its prevalence varies according to the region and population studied.
Higher prevalence in adolescents and young adults (10–40 years).
High incidence in adolescents of African descent in the USA and in populations in Brazil and Korea.
An increase in severe cases has been found in the paediatric population.
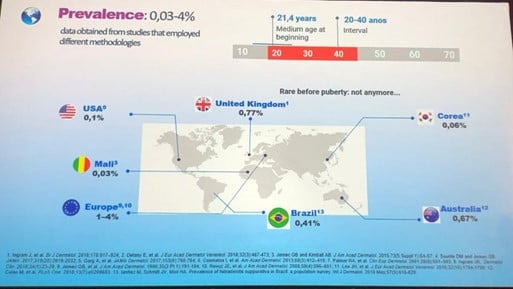

Diagnosis often delayed (>12 years), despite family history in one third of cases.
Patients often see multiple doctors before the correct diagnosis is made.
Presence of firm, inflamed nodules (<1 cm), often difficult to recognise in early stages.
Painful fluctuating abscesses, sometimes mistaken for boils.
Tunnels (dermal or subcutaneous fistulas) which may be numerous, draining or dry, with multiple orifices.
Residual lesions: atrophic scars and double comedones
Typical anatomical distribution: armpits, groin, gluteal region, breast region.
It is important to differentiate HS from superficial folliculitis: the latter does not leave atrophic scars or characteristic comedones.
In advanced cases, it may coexist with dissecting cellulitis of the scalp.
HS is a progressive disease: over time, lesions spread and tissue damage accumulates.
Pain is a cardinal feature, along with occasional pruritus.
Progression has been documented in two thirds of patients at four-year follow-up.
The impact is not only physical; it significantly affects quality of life, productivity and interpersonal relationships.
Importance of early diagnosis
There is a “window of opportunity” where early diagnosis and treatment can limit progressive damage.
Skin ultrasound can be a useful tool to improve diagnostic accuracy.
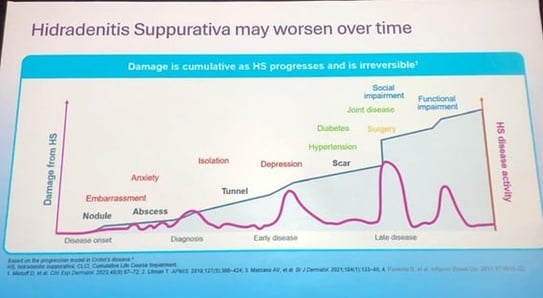
Hidradenitis suppurativa is a severe, under-diagnosed follicular inflammatory disease that can begin in adolescence and progress rapidly if not recognised early. Clinical diagnosis should take into account lesion morphology, lesion distribution and recurrence. Given its cumulative physical and psychosocial impact, increasing medical awareness is essential, particularly in the field of paediatric dermatology, as is ensuring appropriate follow-up and treatment from an early stage.
Speaker: Dr Virginia Ruth Lopez Gamboa (Argentina)
Hidradenitis suppurativa (HS) is a chronic, recurrent and progressive inflammatory disease of the hair follicle, with an auto-inflammatory basis and a significant physical and psychosocial impact. Understanding its pathogenesis is essential to improve diagnostic and therapeutic strategies, especially in early forms of the disease, including the paediatric population.
Fundamental aspects of the pathogenesis of HS
HS is not an infection. It is not due to lack of hygiene and is not determined by race.
It is a complex, systemic, autoinflammatory disorder with immune dysregulation and altered stem cell fate.
1. Genetic theory
- Complex inheritance, with involvement in 50–70% of cases (mostly women)
- Some mutations detected:
- Gamma-secretase genes (NOTCH pathway)
- Polymorphisms in TNF, IL-1 and IL-6 receptors
- SOX9, a transcription factor in hair follicle stem cells, is key:
2. Immunocellular theory
- It involves cells such as:
- Dendritic cells, Th1 and Th17 lymphocytes, neutrophils and plasma cells
- Excessive secretion of pro-inflammatory cytokines
- Involvement of fibroblasts and keratinocytes in chronic phases: fibrosis and aberrant scarring
- These mechanisms are the targets of currently approved biological therapies
3. Hormonal theory
- Incomplete X chromosome inactivation in females has been proposed as a cause of immune dysfunction.
- Androgen-regulated SOX9 is altered in this context.
- Adiposity increases the conversion of androgens to oestrogens, modulating inflammation.
- This explains the use of drugs such as spironolactone, finasteride or metformin in some cases.
The traditional model suggests that the follicular plug initiates the process.
Current evidence suggests that dermal inflammation precedes plugging, creating a vicious circle.
The process includes: inflammation → plugging → rupture → more inflammation → tunnelling and scarring.
HS is a multifactorial disease, with interrelated genetic, immunological and hormonal mechanisms.
Early identification of mild forms, especially in the paediatric population, can prevent progression and permanent sequelae.
SOX9 emerges as a central molecule in pathogenesis.
It is essential to personalise the therapeutic approach, taking into account triggering factors and the clinical stage.
The primary triggering mechanism is still unknown and further clinical and translational research is needed.
Speaker: Dr Ximena Wortsman (Chile)
Hidradenitis suppurativa is a chronic, devastating and underdiagnosed inflammatory disease involving hair follicles in intertriginous areas. Historically, it was considered to be a disease of the apocrine glands, but recent evidence has shown that its origin is related to follicular disruption. Skin ultrasound has emerged as a key diagnostic tool for early disease detection, disease activity assessment, treatment guidance and surgical planning.
1. Advantages of high-frequency skin ultrasound
It enables viewing skin layers, hair follicles, glands and fistulous tracts in detail.
It has better axial spatial resolution than CT or MRI scans for skin structures.
It detects abnormalities that are not clinically visible, enabling earlier diagnosis.
2. New findings in the pathophysiology of HS
The disease originates in the hair follicles, not in the apocrine glands.
Follicular rupture contributes to the formation of fluid collections and inflammatory tunnels.
Fragments of keratin and hair shafts have been found to be retained within these tunnels, which is associated with increased severity.
3. Ultrasound protocol and diagnostic criteria
There are standardised protocols for different anatomical regions.
Ultrasound criteria include: cystic structures, fluid collections, fistulous tracts and changes in echogenicity.
The ultrasound classification has been updated to include sub-stages (3A and 3D) and number of affected regions.
4. Value of ultrasound as a staging tool and biomarker
Useful for accurate determination of the degree of active inflammation.
It enables differentiating HS from its simulators (such as abscesses or recurrent folliculitis).
In more than 80% of cases in adults (and 90% in children), the use of ultrasound changed therapeutic behaviour.
5. Monitoring and evaluation of treatment response
It enables changes in echogenicity, tunnel collapse and decreased vascularity with Doppler to be assessed after treatment.
Useful for monitoring the effectiveness of biological treatments, antibiotics and photodynamic therapy.
6. Surgical planning and guided procedures
Improved delineation of surgical margins, which may reduce recurrences.
It guides intralesional corticosteroid injections, ensuring accuracy and avoiding adverse effects.
7. International validation of the use of ultrasound in HS
Recent Chilean guidelines and international consensuses validate the use of ultrasound as part of the diagnostic and therapeutic algorithm for HS.
More than 95% agreement was achieved on the items evaluated (diagnostic criteria, staging, monitoring, guided procedures and usefulness in research).
High-frequency skin ultrasound is a validated, reproducible and highly effective tool for diagnosis, staging, therapeutic monitoring and procedural planning in patients with hidradenitis suppurativa. Its use should be established as a standard of care, especially due to its ability to detect disease at early stages, guide clinical decision-making and optimise multidisciplinary treatment.
Speaker: Dr Jacek Szepietowski (Poland)
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease characterised by painful, recurrent and debilitating lesions mainly affecting the intertriginous areas. Although its clinical presentation is well known, the subjective and psychosocial burden associated with the disease is often underestimated. This talk addressed the emotional, social and psychological consequences of HS, especially in paediatric and adolescent populations, highlighting the need for an interdisciplinary approach to improve patient quality of life.
HS manifests with visible skin lesions as well as with subjective symptoms such as severe pain.
Pain correlates directly with clinical severity according to the Hurley and IHS4 scales.
More than 80% of patients report significant pain.
Patients perceive HS to be a “miserable”, “unbearable” disease that destroys physical and emotional well-being.
It has a profound impact on quality of life, affecting physical and mental health, social life and self-esteem.
There are multiple validated tools for assessing quality of life in HS, although many are validated only in the adult population.
Quality of life decreases proportionally with the number of areas affected and the seriousness of the clinical stage.
In a small number of studies in adolescents with HS, a higher prevalence of anxiety, depression and alexithymia (difficulty identifying and expressing emotions) has been observed.
Problems of passive self-destructiveness, demotivation, and problematic substance use have also been identified.
HS causes marked social stigma, comparable to other visible dermatoses such as atopic dermatitis or alopecia.
This stigma affects social integration, interpersonal relationships and self-perception.
HS has a significant impact on sexuality, especially in adolescents who are just beginning their sex lives.
High levels of sex life dissatisfaction and difficulties in establishing intimate relationships have been reported.
There is a strong correlation between low life satisfaction, anxiety, depression and perception of disease management.
A good doctor-patient relationship based on trust can significantly improve therapeutic outcomes.
The need for effective and personalised treatments was emphasised.
An interdisciplinary approach was recommended, including dermatologists, rheumatologists, psychologists and psychiatrists.
Psychological support is key to improving adjustment to the disease and quality of life.
HS is much more than a skin disease; it represents a major physical, emotional and social burden. Patients need to be heard not only regarding their clinical symptoms, but also regarding their personal experiences. Comprehensive care should contemplate clinical management, psychosocial support and improving self-esteem and functioning. As doctors, our role must go beyond dermatological treatment and encompass a holistic view that accompanies patients from suffering to stabilisation and well-being.
Speaker: Dr Renata Magalhaes (Brazil)
Hidroadenitis suppurativa (HS) is a chronic, debilitating inflammatory disease. Its manifestation in the paediatric population represents a diagnostic and therapeutic challenge. Although it has traditionally been considered to be a young adult disease, early onset cases are increasingly recognised, even before the age of 12 years. This highlights the need for specific and timely therapeutic approaches in this age group.
In a centre of excellence with more than 200 HS patients, 15% reported symptoms before the age of 12 years, and another 15% between the ages of 12 and 18 years.
The disease in adolescents tends to have a more aggressive course with a significant impact on quality of life.
Case reports showed childhood onset with severe progression, including one that progressed from mild lesions to severe disease in seven years.
Identifying these associated conditions enables more comprehensive and earlier intervention.
Early diagnosis and treatment are essential to modify the course of the disease.
Delaying the initiation of biologic treatments can significantly decrease the response rate.
An Italian study showed that a delay of more than 10 years is associated with double the risk of treatment failure.
Useful in early stages or mild disease
Options: topical clindamycin, resorcinol (although not officially approved), zinc supplementation (empirical use)
First-line treatment for moderate-to-severe cases
They include cephalexin, doxycycline (from age 8), clindamycin and rifampicin (with caution).
Mainly considered in cases with coexisting severe acne
Isotretinoin is useful, although with variable results; acitretin may be considered in specific cases.
Limited evidence, but may be considered in cases with signs of hyperandrogenism or insulin resistance
They represent the most evidence-based option for patients aged ≥12 years.
Main agents used:
Surgery may be considered in selected patients, ideally after stabilisation with medical treatment.
Example: 15-year-old patient with right axillary involvement managed with biologics followed by surgical excision with good results.
Laser and light therapies (CO₂ laser, Nd:YAG) may be useful as an adjunct.
Essential multidisciplinary management: dermatology, rheumatology, psychology, nutrition.
The treatment of hidradenitis suppurativa in the paediatric population represents a unique window of opportunity to modify the course of the disease and improve long-term prognosis. Early recognition, comprehensive management of comorbidities, and timely initiation of effective therapies, including biologics, are fundamental pillars of the current approach. While there are still limitations in the evidence, especially in children under 12 years of age, new guidelines are starting to include specific recommendations for this age group. This marks important progress in treatment personalisation.
Speaker: Dr Mario Chaves Cirujano (Brazil)
Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit, which is often underdiagnosed, delaying appropriate treatment. In children and adolescents, the delay in surgery compromises prognosis and perpetuates progression to more severe forms. This talk addressed the importance of timely surgical intervention, the therapeutic options available, and the role of complementary technologies in optimising outcomes.
Surgery should be considered early, even in adolescents, to prevent progression to advanced stages (Hurley III).
The decision to perform surgery should be based on the extent, location and severity of HS and the patient’s comorbidities.
Not interfering with their school calendar is essential in children and adolescents.
Removal of inflammatory masses, keratotic debris and chronic granulation tissue
Control of the infection and reduction of the risk of recurrence
Incision and drainage of abscesses
Deroofing and unroofing (tunnel roofing and unroofing techniques)
Wide excision with 1–2 cm lateral margins and flap or graft coverage
Primary closure where possible, especially in localised lesions
Haemorrhage, infection, hypertrophic scarring, retraction, dehiscence and recurrence
CO₂ laser: useful for chronic lesion ablation or excision with minimal thermal injury and better visualisation Promotes secondary intention healing.
Negative pressure (vacuum): indicated for high-risk surgical incisions; reduces the risk of infection and dehiscence
Paediatric clinical examples:
Cases in adolescents with axillary, inguinal and perineal lesions treated with wide excision and reconstruction with V-Y flaps or primary closure
Satisfactory functional and aesthetic postoperative results
Surgery is a fundamental tool in the treatment of hidradenitis suppurativa, especially in young patients. Early surgery can slow disease progression, reduce inflammatory burden and improve quality of life. Adopting a multidisciplinary approach is key, taking into account the mental health, psychological support and social context of paediatric patients. The use of technologies such as CO₂ laser and negative pressure therapy optimises surgical outcomes and minimises complications. Early, well-planned and personalised surgical treatment can radically transform the prognosis of HS patients.
Coordinator: Dr Catherine C. McCuaig (Canada)
Co-Coordinator: Dr Ana María Saénz (Venezuela) & Dr Josaine Sanjinés (Bolivia)
Speaker: Dr Josaine Sanjinés (Bolivia)
The pathophysiology, epidemiology, clinical manifestations and current treatment options for molluscum contagiosum and warts caused by human papillomavirus (HPV) were reviewed. Both conditions are common cutaneous viral infections in the paediatric population. Although they are usually self-limiting infections, their treatment can be challenging, especially in multiple, symptomatic or localised cases in visible areas.
Caused by a human-specific poxvirus
It is transmitted by direct contact with infected skin, auto-inoculation or fomites.
Waterborne transmission has not been proven, although it has been associated with activities such as swimming (“water wart”).
Prevalence in US children: approximately 62%
More common between the ages of 0 and 16 years
Incubation period: 2 weeks to 6 months
Pearly, skin-coloured, umbilicate lesions measuring 1 to 10 mm
Koebner phenomenon in trauma areas
In atopic dermatitis, hypersensitivity and extensive dissemination may be found.
Immunosuppression: HIV, transplants, chemotherapy (more extensive and refractory lesions)
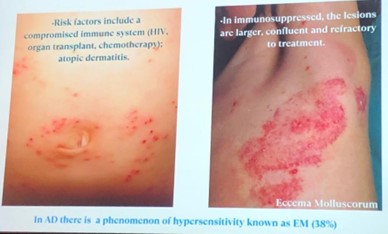
Watchful waiting in non-symptomatic or sparse lesions
The sign of perilesional inflammation may indicate imminent resolution.
References:
Keam SJ. Berdazimer Topical Gel, 10.3%: First Approval. Drugs. 2024 Mar;84(3):363-368. doi: 10.1007/s40265-024-02012-9. PMID: 38409574.
Paller AS, Green LJ, Silverberg N, Stripling S, Cartwright M, Enloe C, Wells N, Kowalewski EK, Maeda-Chubachi T. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol. 2024 May-Jun;41(3):438-444. doi: 10.1111/pde.15575. Epub 2024 Feb 27. PMID: 38413239.
Common warts: hyperkeratotic lesions on the face and extremities
Plantar warts: smooth surface with dark spots (thrombosed capillaries)
Condylomata acuminata: in the genital and perianal region
Oral warts: on the lips, gums, palate or tongue
Both molluscum contagiosum and viral warts are common, benign, self-limiting infections in childhood. The choice of therapy must be personalised, taking into account the patient’s age, wart location, symptoms, number of lesions and immune status. There are multiple approved options, but watchful waiting is also valid in many cases. Communication with parents and appropriate follow-up are key to successful and safe treatment.
Speaker: Dr Catherine C. McCuaig (Canada)
In recent years, the topical treatment of inflammatory skin diseases such as atopic dermatitis and psoriasis has evolved considerably. New molecules with innovative mechanisms of action have been approved, notably phosphodiesterase-4 (PDE-4) inhibitors, Janus kinase inhibitors (JAKi) and aryl hydrocarbon receptor (AHR) agonists. These therapies promise to significantly improve clinical management, particularly in the paediatric population. However, their high cost and limited availability continue to be major barriers.
1. PDE-4 inhibitors
2. Topical JAK inhibitors
Assessed in hand eczema and paediatric linear morphea
Adverse events: local irritation (<1%), some follicular KP-like reactions
3. Aryl hydrocarbon receptor (AHR) activators
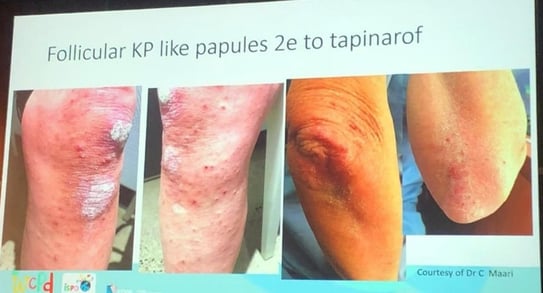
4. Clinical and comparative considerations
Short studies (28 days), with limited data on AHR activators
New topical therapies offer valuable and effective alternatives for the treatment of atopic dermatitis and psoriasis, including in the paediatric age group.
Current challenges: lack of head-to-head comparative studies, limited evidence on treatment combinations and high cost. The future looks bright with innovative molecules that could redefine the topical treatment of chronic inflammatory diseases in paediatric dermatology.
Speaker: Dr Ana María Saénz (Venezuela)
Epidermolysis bullosa (EB) is a group of rare genetic diseases characterised by extreme fragility of the skin, causing blistering and sores with minimal trauma. Treating this pathology is a major challenge, especially in resource-limited countries, as it requires a multidisciplinary approach. During the Paediatric Dermatology Congress, new developments in innovative topical treatments were presented, from gene therapies to accessible natural solutions.
Example: gene-corrected keratinocyte autografts have shown ulcerative recurrences after a few years, showing that optimisation is still required.
Inaccessibility for many Latin American countries
Topical treatment in EB has progressed significantly, offering new hope, especially with the approval of the first in vivo gene therapy. However, most of these therapies are inaccessible to many regions due to their high cost. Alternatives such as birch bark extract are an effective and feasible option in resource-limited contexts. Continuing to promote research, international collaboration and comprehensive patient support is essential in order to achieve a more equitable and effective approach to this complex disease.
Speaker: Dr Irene Lara Corrales (Canada)
This talk addressed topical treatments approved over the last five years for the management of acne vulgaris and hidradenitis suppurativa (HS). These pathologies share similar pathophysiological mechanisms—mainly follicular occlusion and secondary inflammation—which justifies their joint analysis. While multiple systemic therapies are available, optimising topical options is key, especially for patients with mild disease or as part of combination or maintenance strategies.
It has been shown not to generate significant systemic exposure, unlike its oral formulation.
Two phase III trials confirmed its efficacy in reducing inflammatory and non-inflammatory lesions, with good tolerability and safety after 52 weeks.
Included in new treatment guidelines with high recommendation and moderate level of certainty
First topical anti-androgen approved for acne; it inhibits dihydrotestosterone (DHT) action at the level of androgen receptors in sebaceous glands.
Studies in adolescents and adults have shown efficacy and a favourable safety profile, although its use may be limited by its high cost.
Conditional recommendation with high level of evidence in the new guidelines
Topical combination that improves the tolerability of benzoyl peroxide
Effective for moderate-to-severe acne, comparable or superior to other combinations
Strong recommendation with moderate certainty in guidelines
First three-in-one topical therapy approved for acne
Studies in adults and adolescents have shown superiority over vehicles and dual combinations, with good tolerability.
Not yet included in the guidelines due to its recent approval
There are currently no approved topical treatments specifically for HS, but trials are ongoing:
American guidelines for the medical treatment of HS, including specific recommendations for the paediatric population, have recently been published.
In acne, the development of new topical formulations has expanded the therapeutic arsenal with more specific, effective and safer products.
Although cost and adherence continue to be clinical challenges, these advances enable personalised treatment stratification.
For hidradenitis suppurativa, there is still a shortage of topical therapies with solid evidence, although current studies are promising.
Staying up-to-date with future approvals and the results of ongoing clinical trials is essential.
Speaker: Dr Felipe Enrique Valderrama (Peru)
Alopecia areata and vitiligo are chronic autoimmune inflammatory diseases that commonly affect the paediatric population. Although clinically different, they share common pathogenic mechanisms and similar therapeutic challenges. This presentation addressed the most relevant topical therapeutic options in children, with emphasis on JAK inhibitors as a promising new alternative.
Autoimmune disease with genetic predisposition
It can have a mild, moderate or severe course (totalis, universalis, diffuse).
Clinical diagnosis supplemented by trichoscopy: black dots, exclamation mark hairs, broken hairs
Severity assessment using the SALT score.
Topical and intralesional corticosteroids: first line, safe in children
Prostaglandin analogues: (bimatoprost, latanoprost) for scalp and eyelashes, although with limited evidence
Topical JAK inhibitors (tofacitinib, ruxolitinib): emerging option when steroids fail
Topical minoxidil: useful adjuvant
Other methods:
In extensive cases (>50%): systemic therapy with corticosteroids or immunomodulators (methotrexate among others)
10-year-old girl with extensive alopecia areata, refractory to steroids and methotrexate
Successful treatment with tofacitinib 5 mg twice daily: 60–70% improvement in SALT score within a few months
Increased frequency of segmental vitiligo
High association with atopy and family history of autoimmune diseases
Classification: segmental, non-segmental, mixed or unclassified
Multiple mechanisms: genetic predisposition, autoimmunity, oxidative stress and neurogenic theories
Convergent theory: unification of hypotheses explaining the loss of melanocyte viability
Indicated when <10% of the body surface area is affected
Topical corticosteroids: mometasone or fluocinolone used intermittently to minimise local adverse effects
Calcineurin inhibitors: tacrolimus or pimecrolimus, especially on the face and sensitive areas
Topical JAK inhibitors: FDA-approved ruxolitinib cream (paediatric use still under study)
Paediatric patient with vitiligo on her upper lip, treated with topical ruxolitinib and phototherapy, with 80% repigmentation in 2 months
In widespread cases: Narrowband UVB phototherapy: most commonly used method
Surgery (suction blister epidermal grafts): option for refractory areas (lips, face)
Both alopecia areata and vitiligo share pathogenic mechanisms such as autoimmunity and oxidative stress.
The choice of treatment should be based on clinical assessment and severity.
Topical corticosteroids and calcineurin inhibitors continue to be mainstays of initial treatment.
Topical JAK inhibitors, such as tofacitinib and ruxolitinib, have shown to be promising alternatives due to their efficacy and safety profile in paediatrics.
Combination treatment with phototherapy can enhance therapeutic results.
Speaker: Dr Fernanda Bellodi Schmidt (United States)
Although topical treatments are generally considered safe, especially in paediatrics, their inappropriate use can have serious consequences. This talk aimed to highlight the acute toxicity risks associated with topical medicinal products frequently used in paediatric dermatology. Through real case reports, it was shown how factors such as age, skin barrier status, dose and application form can contribute to significant systemic adverse events.
Children’s skin is significantly different from adult skin:
Immature stratum corneum until 2 to 5 years of age
Thinner epidermis and lower lipid content
Higher body surface/weight ratio
These characteristics favour increased percutaneous absorption and risk of toxicity.
1. Topical anaesthetics (lidocaine/prilocaine – EMLA®, lidocaine – LMX®)
2. Alcohols and antiseptics
3. Hydrogen peroxide
4. DEET (diethyl-meta-toluamide, topical insecticide)
5. Salicylic acid
Speaker: Dr Lisa Weibel (Switzerland)
During this presentation, recent advances were shared in paediatric dermatology focusing exclusively on clinical observations, excluding therapeutic and genetic aspects. The talk was based on a selection of ten scientific publications highlighting new clinical entities, unusual phenotypic patterns and relevant associations with diagnostic implications. The most important findings are described below.
1- Segmental corymbiform congenital melanocytic naevi
2- Orthostatic vascular syndrome in children
a. Urticarial lesions with erythrocyanosis alternating with anaemic macules when a patient stays in an orthostatic position for some minutes
b. A Canadian study reported 42 cases; predominance in girls, frequently associated with ADHD (50% of cases) and dysautonomia symptoms (60%) such as intolerance to orthostatic position. Cases have also been described in infants.
c. Beta-blockers and antihistamines did not show good response. Its course is benign; spontaneous resolution in most cases in 3 to 4 years
3- Voriconazole phototoxicity with xeroderma pigmentosum phenotype
a. Cases in immunocompromised patients, including children with Down’s syndrome treated with voriconazole.
b. Voriconazole can cause phototoxicity, but has also been linked to a high risk of aggressive squamous cell carcinoma and even melanoma in both children and adults.
c. Patient skin phenotype is remarkably similar to that seen in individuals with xeroderma pigmentosum (XP).
d. It was shown that the drug does not directly modify the expression of genes involved in DNA repair (as in XP), but reduces repair by altering chromatin density, which prevents the repair system from accessing damaged DNA.
e. This adverse voriconazole effect can be reversed by the concomitant administration of histone deacetylase inhibitors, such as valproic acid, ribostatin, etc.
References:
AK Haylett, S Felton, DW Denning, LE Rhodes.Voriconazole‐induced photosensitivity: photobiological assessment of a case series of 12 patients British Journal of Dermatology, 2013
Alberdi Soto, M., Aguado Gil, L., Pretel Irazabal, M., Bonaut Iriarte, B., Irarrázabal Armendariz, I., Lera Imbuluzqueta, J. M., … Ivars, M. (2014). Accelerated photoaging induced by voriconazole treated with Q-switched Nd:YAG laser: Case report and review of the literature. Journal of Cosmetic and Laser Therapy, 16(6), 314–316. https://doi.org/10.3109/14764172.2014.957215
Miller DD, Cowen EW, Nguyen JC, McCalmont TH, Fox LP. Melanoma Associated With Long-term Voriconazole Therapy: A New Manifestation of Chronic Photosensitivity. Arch Dermatol. 2010;146(3):300–304. Doi:10.1001/archdermatol.2009.362DD Arch Dermatol 2010
CowenEW, Nguyen JC, Miller DD, et al.Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol 2010;62:31–7.
4- How many lesions are too many? Clinical thresholds for genetic assessment.
a. When present in large numbers, we know that some skin lesions may be associated with underlying diseases. However, for many of these lesions, the numerical threshold that should alert us is not well defined.
Suggestions for suspecting systemic diseases based on the number of lesions:
5- MiTES (Mid-facial Toddler Excoriation Syndrome)
a. Self-inflicted ulcers in the centrofacial region in children under 1 year of age, with symmetrical excoriations in an “X” pattern
b. Self-limiting course (approximately 5 years), leaving atrophic scars. Differentiate from congenital insensitivity to pain (CIP), in which touch, pressure and temperature are normal, but no pain is perceived.
c. Both associated with variants in the PRDM12 gene; they differ in the expansion of polyalanine repetitions in this gene
6- Transient infantile lingual leukoplakia
a. Non-removable whitish plaque on the dorsal tongue, sparing the tip of the tongue
b. Not associated with Candida or systemic pathologies
c. Spontaneous resolution on initiation of solids
Reference:
Lam JM, Schwieger-Briel A, Nguyen T, Torrelo A. Transient infantile lingual leukoplakia: An underrecognized cause of white tongues in infancy. Pediatr Dermatol. 2024 May-Jun;41(3):476-479. doi: 10.1111/pde.15576. Epub 2024 Feb 27. PMID: 38413200.
7- Verrucous epidermal naevus in Blaschko pattern related to PTEN
a. Patient with linear verrucous naevus, macrocephaly, developmental delay (cortical dysplasia on MRI) and cataracts. Biopsy showed a sebaceous naevus but the genetic study enabled diagnosing a germline variant in the PTEN gene.
b. Diagnosis: PTEN hamartomatous syndrome, presenting as mosaicism. The naevus may be called linear PTEN naevus or also linear Cowden naevus. The main characteristic of this naevus is that it is very verrucous and papillomatous and has a hyperkeratotic component.
Keep in mind that a linear verrucous epidermal naevus associated with macrocephaly may have a PTEN mutation, and should therefore be monitored and followed up with oncological surveillance protocols.
8- Epidermolytic epidermal naevus: biopsy or no biopsy?
a. Low risk of transmission of epidermolytic ichthyosis in parents with extensive naevi
b. Genetic counselling is very complicated in this context because the risk of transmission varies enormously, from 0% if no gonadal cells are affected, to 50% if all gonadal cells are affected in one of the parents.
It is also difficult because the current techniques to investigate gonadal involvement are either not feasible (e.g. in women) or do not reliably determine the degree of gonadal involvement.
c. Biopsy is recommended in extensive lesions to assess risk and consider genetic counselling.
9- Segmental stiff skin syndrome
a. Skin hardening since childhood, with hypertrichosis and joint involvement (joint pain or restriction of movement, due to involvement of underlying fasciae and joints)
b. Clinical differential diagnosis with localised scleroderma (has adnexal atrophy) and connective naevus
c. Diagnosis requires clinical/histological findings and imaging studies.
10- How to take the best scabies sample: under Wood’s light
Traditionally, in polarised dermoscopy, the characteristic sign of scabies is the “delta sign”, representing the anterior parts of the Sarcoptes scabiei mite (head and forelegs). However, this method does not always enable visualising the mite’s entire body.
With the addition of UV dermoscopy (using 365 nm light), the authors found that the mite’s entire body emits a bright fluorescence, appearing as an oval or spherical structure. This finding has been dubbed the “ball sign”. This technique improves mite visualisation by eliminating interferences such as skin scales, thereby enabling the parasite to be more clearly identified.
Reference:
Aslan Yürekli. A new sign with UV dermoscope in the diagnosis of scabies: Ball sign. Skin Res Technol. 2023;29:e13336
These recent clinical findings underline the importance of close dermatological observation, especially in paediatrics. Several of the patterns described have important diagnostic, prognostic and genetic implications and should be considered in daily clinical practice. When necessary, the clinical approach should be complemented with histological and molecular studies to achieve an accurate diagnosis and guide the appropriate follow-up of these patients.
Speaker: Lawrence Eichenfield (United States)
Therapeutic update in paediatric dermatology: recent advances in atopic dermatitis, psoriasis and alopecia areata
In recent years, remarkable progress has been made in the development and approval of dermatological treatments in the paediatric population, especially for chronic and inflammatory diseases such as atopic dermatitis, psoriasis and alopecia areata. This presentation reviewed recent findings from multicentre clinical trials and extension studies that have expanded the therapeutic arsenal for these patients, highlighting both topical and systemic treatments and their clinical impact beyond skin management.
Non-steroidal topical treatments:
Systemic treatments:
Paediatric dermatology is undergoing a therapeutic transformation, with significant clinical advances driven by international collaborations. New non-steroidal topical agents and targeted systemic therapies have improved both skin management of diseases such as AD and psoriasis, as well as systemic comorbidities and quality of life. The importance of continuing education for paediatricians and healthcare professionals on the up-to-date treatment of these pathologies was highlighted.
Speaker: Dr Brigitte Dréno (France)
This presentation addressed advances in the understanding of the skin microbiome and its key role in skin homoeostasis and in inflammatory, infectious and neoplastic diseases. The speaker highlighted the dynamic balance between commensal and pathogenic bacteria, the influence of the skin microenvironment on bacterial virulence, and advances in bacteriotherapy as an emerging therapeutic approach.
Any chronic disturbance (such as UV damage) can cause dysbiosis, with loss of bacterial diversity and increased virulence profiles in previously commensal bacteria.
Bacterial extracellular microvesicles
Example: S. epidermidis stimulates secretion of antimicrobial peptides and promotes healing.
Imbalance or dysbiosis delays healing
The microbiome can counteract UV-induced immunosuppression.
Use of probiotics and prebiotics to modulate immunity, skin barrier and inflammation
Inhibits epidermal lipid synthesis and decreases antimicrobial peptides
With age, diversity is lost and chronic low-grade inflammation increases.
The skin microbiome is a dynamic ecosystem that is essential for skin homoeostasis. Commensal bacteria are involved in the defence against pathogens and also modulate inflammation, healing and even skin cancer prevention. New therapeutic strategies—such as bacteriotherapy, the use of extracellular vesicles and modulation of the microenvironment—open up a promising field in dermatology, both as regards the prevention and treatment of inflammatory and neoplastic diseases. The key is to maintain the diversity and balance of the microbiome.
Reference:
Lee YB, Byun EJ, Kim HS. Potential Role of the Microbiome in Acne: A Comprehensive Review. J Clin Med. 2019 Jul 7;8(7):987. doi: 10.3390/jcm8070987. PMID: 31284694; PMCID: PMC6678709.
C. Cheung, C. Mias, U. Lancien, S. Corvec, V. Mengeaud, A. Khammari, H. Duplan, B. Dreno,
333 Different proinflammatory profiles of Cutibacterium acnes IA1 extracellular vesicles from healthy and acne skin: modulation by Myrtacine and Celastrol, Journal of Investigative Dermatology,Volume 144, Issue 12, Supplement,2024,Page S285
Cros MP, Mir-Pedrol J, Toloza L, et al. New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders. Sci Rep. 2023;13:16058. doi:10.1038/s41598-023-43354-w
Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell. 2019;176(5):998–1013.e16. doi:10.1016/j.cell.2018.12.040.
Speaker: Dr Ilona Frieden (United States)
ISSVA Classification Update (2024)
Capillary malformations - Subtypes highlighted
Clinical significance of the classification
Reference: https://vascern.eu/group/vascular-anomalies/clinical-decision-support-t…
Reference: Borst A. Targeted medical therapies for vascular anomalies. Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):709-717. doi: 10.1182/hematology.2024000599. PMID: 39644074; PMCID: PMC11665586.
Reference:
Bradley, Flora et al. A retrospective multicenter cohort study of differences in clinical characteristics of Infantile Hemangiomas in preterm and term infants: Prematurity increases risk of permanent cutaneous sequelae. JAAD 2025, Volume 92, Issue 3, Pages 546-553. doi: 10.1016/j.jaad.2024.09.066
Failure of beta-blocker treatment