2 professionals
Bioderma Congress Reports European Hair Research Society - EHRS 2025
Bioderma Congress Reports European Hair Research Society - EHRS 2025
Get access to exclusive dermatological services to increase your professionnal knowledge: +500 pathology visuals, clinical cases, expert videos
Benefit from valuable features: audio listening, materials to be shared with your patients
Stay informed about the upcoming events and webinars, latest scientific publications and product innovations
Already have an account? login now
Reports written by Dr Lidiya Todorova (Dermatologist, Bulgaria) & Dr Nicolas Kluger (Dermatologist, Finland)

Related topics
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Prof. Lidia Rudnicka, Poland
Trichoscopy is a non-invasive diagnostic technique, to visualize specific components of the scalp not seen with the naked eye. Key trichoscopic structures include hair shafts, follicular openings (named “dots”), skin surface and blood vessels. Each of which provides valuable clues for the diagnosis of hair and scalp conditions like alopecia areata, androgenetic alopecia, and scarring alopecias.
Fundamental abnormalities related to the hair shafts seen in trichoscopy include:
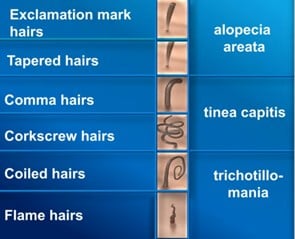
Probably the most famous hair shaft abnormality is the “exclamation mark hair”. It is characterized by a normal distal ending and thinner proximal one, which is a sign of low mitotic activity of the cells. In trichoscopy, the hair shaft abnormalities can be used as a valuable diagnostic tool for different hair diseases:
The second important structure in trichoscopy is the hair thinning, which can be synchronized (all hair shafts are with the same diameter), typical for telogen effluvium, or unsynchronized (variation of the hair shaft diameter, anisotrichia) – characteristic for androgenetic alopecia.
In terms of the number of hairs per follicular unit, it is normal when one hair follicle includes from 1 to 3 hair shafts. In androgenetic alopecia for example, there is a predominance of 1 hair per unit, while in tufted folliculitis the hair shafts are more than 5 in follicular unit.
In trichoscopy, the follicular openings lacking the hair shafts are called “dots”. “Yellow dots” are empty hair follicles filled with sebum and/or keratotic material, if multiple and regularly distributed, are typical for a long-lasting alopecia areata. “Black dots” mark the hair follicles filled with hair shaft residue after the hair shaft was destroyed or broken (found in active alopecia areata, trichotillomania, tinea capitis).
When looking at the scalp in trichoscopy, we also must evaluate the skin surface and judge whether the changes are diffuse or affecting only the perifollicular area. Diffuse scaling, for instance, is found in psoriasis, seborrheic dermatitis and discoid lupus; while perifollicular one is typical for lichen planopilaris, frontal fibrosing alopecia and folliculitis decalvans.
At last, but not least, blood vessels are also important for the evaluation of the condition. Telangiectatic vessels, making separate islands of erythema on the scalp, may be an indication for an emerging hair disorder. Thick arborized vessels are commonly found in lupus and lichen planopilaris, while thin and regularly distributed arborizing vessels may be a normal finding.
Trichoscopy has become an essential tool in the evaluation of hair and scalp disorders. Prof. Rudnicka’s pioneering work has helped define and standardize these diagnostic criteria, facilitating earlier and more accurate diagnosis and monitoring of hair loss disorders in clinical practice.
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speaker: Marta Kurzeja, Poland
Trichoscopy is a non-invasive, in-office diagnostic technique that uses dermoscopy to examine the scalp and hair. It allows for magnified visualization of the hair shafts and follicles and scalp skin, providing critical insights into various hair and scalp disorders. Trichoscopy may avoid scalp biopsies or allow to choose the best spot for a biopsy, monitor disease progression and treatment response.
Trichoscopy involves the use of a handheld dermatoscope or a videodermatoscope at magnifications typically between 10x–70x. It enables dermatologists to identify specific patterns, such as:
The patient should be warned not to wash the hairs 2-3 days before trichoscopy.
Trichoscopy can be dry or wet (with the application of an immersion oil). Dry trichoscopy allows you to evaluate the presence of scales before wet trichoscopy. A low pressure will be applied when evaluating blood vessels.
In case of diffuse hair loss, the scalp is examined in 3 areas:
Pictures with x20 and x70 magnification are recommended.
In case of patch of alopecia, the area is examined in 2 areas:
Artefacts may include foundation, permanent make up (on the eyebrows), dye in the hair, and dirty dots in children
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speaker: Daniel Asz Sigall, Mexico
Filler-induced alopecia (FA) is a rare form of hair loss that occurs as a complication of dermal filler injections, typically involving hyaluronic acid fillers. It has also been reported after other procedures that involve injections such as deoxycholic acid, fat grafting, poly-L-lactic acid or mesotherapy. It happens when the filler is accidentally injected into or compresses a blood vessel, leading to compromised blood supply in the scalp or forehead, which in turn damages the hair follicles and causes localized hair loss. The mechanism is double: non cicatricial by local vascular compression and cicatricial by vascular thrombosis leading to necrosis. Symptoms develop with day to week after the procedure and may include local erythema, local oedema, pain, ulceration, necrosis and irregular patches of alopecia. Trichoscopy is reminiscent of anagen effluvium, with black dots, broken hairs; tapered hairs; milky red areas and ulcers. In case of suspicion of FA, an ultrasound doppler should be performed to evaluate the extent of the vascular damage. Management includes injection of hyaluronidase (in case of hyaluronic acid injection), injection of triamcinolone, application of warm compresses, oral intake of sildenafil for vasodilation and salicylic acid intake. If treated early, the hair loss can sometimes be reversed. Regrowth will depend on the extent of necrosis. The only risk factor seems to be the quantity injected: injecting less than 1 mL per site is highly suggested to avoid such side effects. With the rise of aesthetic procedures, this side effect will become more frequent.
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Ana-Waskiel-Burnat, Poland
Alopecia areata (AA) is a common, immune-mediated, non-scarring hair loss characterized by the sudden onset of well-demarcated bald patches, most frequently on the scalp but potentially affecting any hair-bearing area of the body. It affects approximately 1–2% of the general population and can present at any age. The condition is believed to result from an autoimmune attack on hair follicles in the anagen (growth) phase, leading to follicular dysfunction without permanent destruction.
Clinically, it may manifest in various patterns, ranging from patchy alopecia to more extensive forms such as alopecia totalis (complete scalp hair loss) or alopecia universalis (total body hair loss). Genetic susceptibility, environmental triggers, and associations with other autoimmune diseases (e.g., thyroiditis, vitiligo) have been implicated in its pathogenesis.
Trichoscopy allows detailed visualization of the features of the disease such as yellow dots, black dots, exclamation mark hairs, and broken hairs. These characteristic patterns aid in early diagnosis, assessment of disease activity, and differentiation from other types of hair loss.
When there is an active hair loss in AA, black dots are commonly seen in trichoscopy. This sign is not pathognomonic, because it is also visible in dissecting cellulitis, trichotillomania and tinea capitis. In disease activity we can also see exclamation mark hairs, which are also present in trichotillomania, tinea capitis and chemotherapy-induced alopecia. These hairs are short, broken strands that are thicker at the tip and narrower at the base, resembling an exclamation mark. Tapered hairs refer to terminal hairs that are longer than exclamation mark hairs, exhibiting a finer proximal end and a thicker distal end. These hairs are visible to the naked eye and are often found around lesions, indicating disease activity. Broken hairs are also a sign of active AA. Pohl-Pinkus’s constrictions, irregular narrowing along the hair shaft, although rarely seen in AA, can indicate active disease or flares, and are also associated with other conditions like chemotherapy-induced alopecia and certain systemic diseases.
When the condition enters a chronic stage, the main trichoscopic markers are the yellow dots and vellus hairs; while in hair regrowth phase are typically observed vellus hairs, circle (pigtail) hairs and upright regrowing hairs. Yellow dots are also seen in androgenetic alopecia, dissecting cellulitis and discoid lupus erythematosus. On the other hand, pigtail hairs are commonly observed in any hair re-growth after, for example, acute or chronic telogen effluvium.
Table 1: Table: Trichoscopy based on disease activity
| Trichoscopic signs in active AA | Trichoscopic signs in chronic AA | Trichoscopic signs in hair regrowth in AA |
|
|
|
|
|
|
|
|
|
|
||
|
Given its unpredictable course, psychological impact, and variable response to treatment, AA remains a subject of ongoing clinical and translational research, with recent focus shifting toward immunomodulatory therapies. As a therapeutic approach, Prof. Waskiel-Brunat mentioned the guidelines of AA treatment given by the European expert consensus statement on the systemic treatment of AA published in JEADV*.
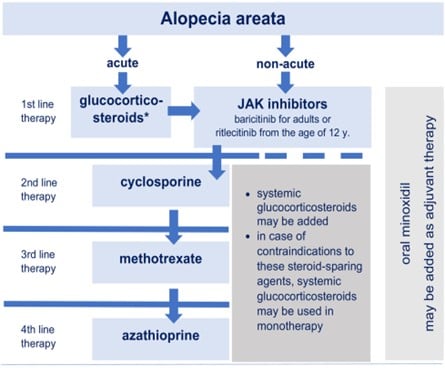
Figure 1. Proposed therapeutic algorithm for the systemic treatment of alopecia areata*
* Rudnicka, L, Arenbergerova, M, Grimalt, R, et al. European expert consensus statement on the systemic treatment of alopecia areata. J Eur Acad Dermatol Venereol 2024; 38: 687-694. https://doi.org/10.1111/jdv.19768
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speaker: David Saceda Corralo, Spain
The SALT score (Severity of ALopecia Tool) is a standardized system to quantify the extent of scalp hair loss in patients with alopecia areata (AA). It was developed by Olsen et al in 1999 (Olsen EA, Hordinsky M, McDonald-Hull S, et al. Alopecia areata investigative guidelines. J Am Acad Dermatol. 1999;40:242–246). The score provides an objective measure to monitor AA progression, assess response to treatment and facilitate clinical trials evaluation
The scalp is divided into 4 regions, each assigned a percentage of total scalp area:
| Scalp Region | % of Total Scalp |
| Vertex (top) | 40% |
| Right profile | 18% |
| Left profile | 18% |
| Posterior (back) | 24% |
For each region, the observer needs to estimate the % of hair loss in that area, then multiply by the region’s weight and sum the results for the total SALT score.
SALT Score = Σ (% hair loss in each region × % region weight)
SALT Score (%) Severity :
JAK inhibitors are currently indicated for moderate to severe AA, with a SALT score of 50 or over.
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Adriana Rakowska, Poland
What is the normal hair: it is when all hair shafts are uniform in thickness, when most of the hair shafts are more than 50 microns in thickness, and when the majority of the follicular units have 3 hair shafts. Androgenetic alopecia (AGA) affects 80% of the male population and 40% of the female population in their lifetime. It is defined by progressive miniaturization of the hair follicle. Due to a genetic predisposition and the activity of dihydrotestosterone (DHT), at each hair cycle the hair shaft becomes thinner and thinner until it is transformed into a vellus hair. Therefore, AGA is characterized by more than 20 percent of hair shaft thickness heterogeneity, also called anisotrichia.
In the trichoscopic picture of AGA there is also the presence of more than 10% vellus hairs and yellow dots. Another marker for AGA is the peripilar sign, which is present due to the perifollicular microinflammation done by the activated T-cells around the upper part of the hair follicles. As mentioned, healthy individuals normally have an average of 3 hairs in a follicular unit, while in AGA the follicular units typically contain single hair.
Since female pattern hair loss (AGA in women) is more complex than male one, there are major and minor criteria for its evaluation.
Major criteria:
Minor criteria:
The diagnosis is made when there are 2 major criteria or 1 major and 2 minor criteria.
The treatment goals in androgenetic alopecia include stopping the progression of the disease as well as improving hair thickness and density. This goal can be reached only with prolonged treatment.
Professor Rakowska highlighted that trichoscopy should be performed every 6 months for treatment evaluation and if necessary, change of therapy. She also suggested the following treatment approach:
| Male AGA / Male Pattern Hair Loss | Female AGA / Female Pattern Hair Loss | |
|
First Line |
Low dose oral minoxidil 1 – 5 mg + Finasteride 1mg OR Dutasteride 0.5mg |
Low dose oral minoxidil 0.5 – 1 mg + Finasteride 2.5-5mg OR Dutasteride 0.5mg + Spironolactone 25-50mg |
| Additional Treatment | Topical minoxidil 2-5%, topical finasteride, Platelet-Rich Plasma, Low Level Laser Therapy | |
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Ozlem Dicle, Turkey
Frontal fibrosing alopecia (FFA) is a primary lymphocytic cicatricial alopecia first described in 1994 by Kossard. It is characterized by progressive frontotemporal hairline recession, often accompanied by eyebrow, eyelash, and body hair loss. It is considered a clinical variant of lichen planopilaris, FFA has seen a dramatic rise in incidence over the past two decades, particularly among postmenopausal women, though increasing reports in men and younger patients suggest a broader demographic spectrum. The condition has a significant negative impact on the quality of life of the affected patients1.
When the FFA is actively progressing, the dermatologist should be aware of the frontotemporal hairline recession, the perifollicular hyperkeratosis and erythema. The hallmark signs of FFA in trichoscopy is called “lonely hairs” found in the affected area. These are an isolated terminal hairs within the alopecic band. On dermoscopic inspection there is also no vellus hairs, but rather empty hair follicles or white dots marking the fibrosing hair follicle.
As mentioned, the condition involves eyebrow loss – common finding in many patients (73-95%)1. In 39% of the cases, eyebrow hair loss precedes scalp hair loss2. Hair loss may also affect sideburns, occipital scalp, eyelashes, body hair, and in men – beard and mustache3,4. When there is sideburn involvement in comparison to the frontotemporal recession, there is less perifollicular scaling and erythema, and prominent transparent proximal hair shaft emergence (79.2%)3.
There are also facial lesions associated with the condition, which dermatologists should be aware of: facial papules, hyper- or hypopigmentation pigmentation, rosacea-like eruptions and prominent facial veins (atrophy of the above covering skin)5.
The recession of the hairline follows several patterns also predictive of the prognosis of the disease:
Prof. Dicle added some non-pathognomonic additional trichoscopic features of the disease:
The therapeutic approach of the dermatologists managing a patient with FFA should have two strength points:
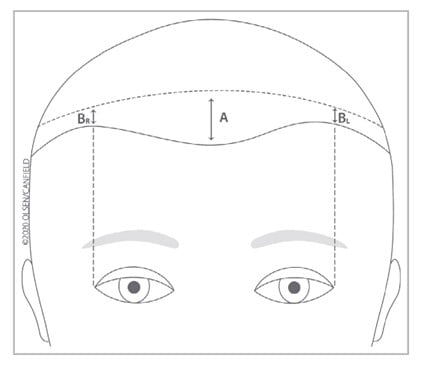
Figure 2. “Quantitative measurement of frontal and temporal hairline recession.
‘A’ is the amount or degree of frontal hairline recession, defined as the distance in centimetres out to one decimal point in the middle of the frontal scalp hairline from the superior edge of wrinkling of the forehead when one raises the eyebrows to an area directly behind this where the hair density is most confluent and homogeneous. This number will be used directly, not categorically, in sequential measurements, ensuring that the most reliable information will be collected for this important feature of FFA. For those light-skinned patients who have had botulinum injections or where an additional aid is needed, the end of photodamage would substitute for the superior edge of wrinkling of the forehead when one raises the eyebrows.
‘B’ is the amount or degree of temporal hairline recession, defined as the distance in centimetres in the middle of each temporal area (defined as the point from the lateral canthus carried superiorly) from the superior edge of wrinkling of the forehead when one raises the eyebrows to an area directly behind this where the hair density is most confluent and homogeneous. For those light-skinned patients who have had botulinum injections or where an additional aid is needed, the end of photodamage would substitute for the superior edge of wrinkling of the forehead when one raises the eyebrows. Measurements of left and right temporal hairline recession should be done separately”1.
To suppress inflammation, the first step is to evaluate whether the inflammatory process is mild, moderate or intense. If mild, then recommendations for therapy include topical steroids one to two times per week and pimecrolimus 1% cream (or tacrolimus 0.1% ointment). Moderate inflammation is managed with the aforementioned topical treatment plus intralesional triamcinolone acetonide 4mg/ml, repeated every 6-8 weeks with/out oral hydroxychloroquine 200mg/daily. When there is intense process, to the topicals could be added intralesional triamcinolone acetonide 4mg/ml, repeated every 6-8 weeks with/out systemic steroids (i.e. intramuscular triamcinolone) plus oral hydroxychloroquine 200 – 400 mg/daily or oral doxycycline 100-200 mg/daily.
To control the progression of the disease, the presenter suggested oral dutasteride 0.5mg/daily or oral finasteride 2.5-5mg daily, for women in reproductive age. Either of these can be combined with topical and oral anti-inflammatory treatments or intralesional triamcinolone acetonide.
For the improvement of the hair thickness, topical minoxidil 2-5% or low dose oral minoxidil 0.5-2mg/daily is suggested. As for the facial papules, the treatment plan includes isotretinoin 5-10mg/daily.
Alternative treatment options should be considered based on the patient’s response to previous treatments and drug accessibility: cyclosporine, methotrexate, pioglitazone, naltrexone, isotretinoin, JAK inhibitors.
Early recognition of FFA is critical for dermatologists, as the condition leads to irreversible follicular destruction. Given its often subtle and slow progression, FFA can be mistaken for age-related hair thinning or other forms of alopecia. Awareness of its distinct trichoscopic and clinical features—such as perifollicular erythema, follicular hyperkeratosis, and the absence of follicular openings—is essential for initiating timely treatment aimed at halting disease progression and preserving hair density.
Literature:
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Maria Fernanda Gavazzoni Diaz, Brazil
Fibrosing alopecia in a pattern distribution (FAPD) is a distinct form of scarring alopecia that presents features of both androgenetic alopecia (AGA) and lichen planopilaris (LPP). Clinically, it mimics patterned hair loss with central scalp thinning, but trichoscopic and histopathologic findings reveal perifollicular erythema, hyperkeratosis, and fibrosis—hallmarks of lymphocytic cicatricial alopecia. Initially described as a variant of LPP, FAPD is increasingly recognized as a separate entity with overlapping features of both inflammatory and hormonally influenced alopecia.
The clinical symptoms of FAPD include:
A very typical clinical picture is the hair thinning with the remaining frontal hair line.
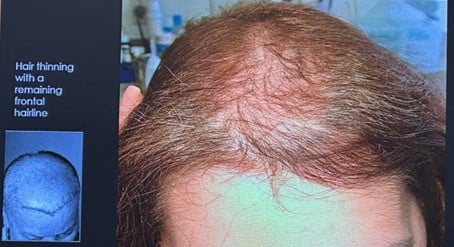
Figure 3. Hair thinning with a remaining frontal hairline, picture taken from the oral communication of Maria Fernanda Gavazzoni Diaz, Brazil.
The trichoscopy shows:
The lecturer also focused on the red island as the newest discovery for FAPD, and describing these as patches of redness with arborizing vessels or red dots and white streaks.
All the mentioned trichoscopic markers can be seen not only on the scalp area but also on the beard. When evaluating a patient with skin of colour, dermatologists should be aware that the dermoscopy of a dark scalp affected by FAPD includes asymmetric and irregular pigmented network, pigmented perifollicular halo, irregularly distributed white dots, absence of follicular ostia and anisotrichia (red islands are rare).
In terms of differential diagnosis between androgenetic alopecia (AGA), FAPD and LPP, here are some valuable strength points:
The suggested treatment of FAPD includes dutasteride 0.5mg/daily or finasteride 1mg/daily (men) and 5mg/daily (women) combined with hydroxychloroquine 400mg/daily and/or doxycycline 100mg/daily plus oral minoxidil 0.25-5m/daily. The other suggested treatment regime includes topical minoxidil 5% combined with topical clobetasol and/or tacrolimus plus intralesional triamcinolone acetonide 25.mg/ml every 6 weeks.
As the prevalence of FAPD appears to be rising, especially in women, heightened awareness is essential for timely intervention and improved patient outcomes. It is crucial for dermatologists to be aware of FAPD, as misdiagnosis as simple AGA can lead to delayed treatment and irreversible hair loss. Unlike AGA, which is non-scarring and slowly progressing, FAPD involves active inflammation. Recognizing subtle clinical signs, using trichoscopy effectively, and confirming diagnosis with biopsy when needed are key to initiating appropriate anti-inflammatory therapies.
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speaker: Alexander Katoulis, Greece
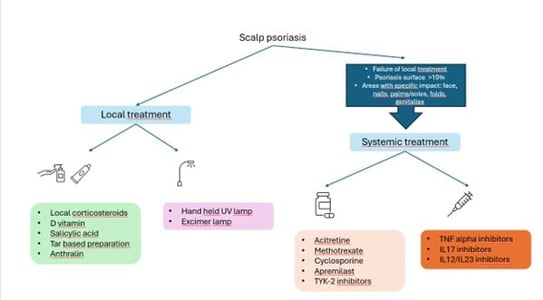
Scalp is among the first affected anatomic sites during psoriasis. It can occur alone or in association with other manifestations. Scalp psoriasis (SPso) affects the occipital and temporal areas. It can stay limited to the hair area or extend beyond on plain skin (forehead, nucha). Itch can be intense. It can also be associated with P. ovale and can be confused or associated with seborrheic dermatitis. Scalp psoriasis as such can have a deep impact on quality of life.
Trichoscopic features of scalp psoriasis include:
Management is summarized here:
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Jacek C. Szepietowski, Poland
Scalp itch, or scalp pruritus, is a common but often underappreciated symptom encountered in dermatology, affecting a wide spectrum of patients with or without visible scalp pathology. It may arise from inflammatory dermatoses such as seborrheic dermatitis, psoriasis, and atopic dermatitis, or occur in the context of hair loss disorders like lichen planopilaris, alopecia areata, and even androgenetic alopecia. In other cases, scalp itch may be neuropathic, psychogenic, or idiopathic, posing a diagnostic and therapeutic challenge.
By definition itch, pruritus, is an unpleasant sensation leading to scratching, a definition proposed in 1660, which is still valid. However, the concept is very imprecise, because “unpleasant” is subjective term. So far there are many new but unsatisfactory attempts to develop a new definition. The itch can be classified as an acute – a defence mechanism, and chronic – a pathological condition. Epidemiologically, pruritus is affecting 8.9-16.8% of the population, and the risk of the development of chronic itch rises by 2% each year. The condition is twice as common in retired people as in professionally active ones. Overall, 11.5% of the patients in dermatology units are hospitalized because of itch and it is the third common reason for hospitalization.
In dermatology itch is often associated with autoimmune connective tissue diseases, autoimmune blistering disease, pregnancy dermatoses, papulo-erythematous disease, allergic conditions of the skin, infections and infestations, skin xerosis, mastocytosis, primary cutaneous amyloidosis, cutaneous lymphomas, disease of eccrine, apocrine and sebaceous glands. It is also common for the dermatologist to encounter patients with no evident cause of scalp pruritus, making it a distressing situation for both the clinician and the patient. Although various pathogenic aetiologies contribute to scalp pruritus, the scalp itself has distinct neuroanatomy and vasculature, specific neuromediators and corresponding receptors, as well as the presence of scalp sebum and microflora, which are all properties that may explain its tendency to be implicated in patients who complain of itch1.
Studies suggest a classification which classifies scalp pruritus into two types: with or without dermatological lesions, and presence or absence of hair loss. Also, it is important to think first about the most common causes and then rule out other, less common aetiologies. The acronym SCALLP and the five steps for scalp evaluation (listen, look, touch, magnify, and sample) are useful tools to keep in mind for an assertive approach in these patients1. Often, the explanation will be related to one of these causes.

Figure 4. SCALLP acronym in order to easily recall the most common causes of scalp itch.
Image source: Vázquez-Herrera NE, Sharma D, Aleid NM, Tosti A. Scalp Itch: A Systematic Review. Skin Appendage Disord. 2018;4(3):187-199. doi:10.1159/000484354
It is also important to remember that scalp itch may be related to more than one cause, and more than one type of lesion may be present. This classification is shown here:
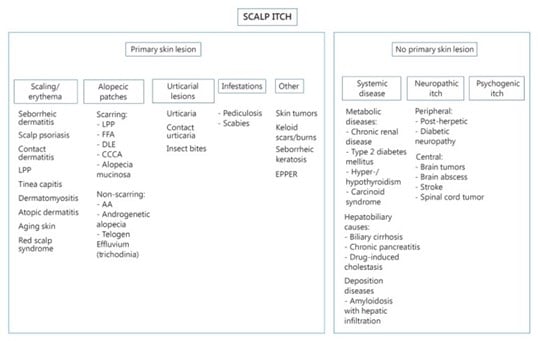
Figure 5. Algorithm for scalp itching based on presence or absence of primary skin lesions. LPP, lichen planopilaris; FFA, frontal fibrosing alopecia; DLE, discoid lupus erythematosus; CCCA, central cicatricial centrifugal alopecia; EPPER, eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy; AA, alopecia areata.
Image source: Vázquez-Herrera NE, Sharma D, Aleid NM, Tosti A. Scalp Itch: A Systematic Review. Skin Appendage Disord. 2018;4(3):187-199. doi:10.1159/000484354
The most common conditions with scalp itch with skin lesions include:
Other more specific lesions that may be diagnosed clinically or with a biopsy are seborrheic keratosis, keloid scars, burns, eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy (EPPER), and rarely, skin tumors1.
Scalp itch (not limited to the scalp) associated with systemic disease can present in the following cases:
Metabolic diseases
Hepatobiliary causes
Paraneoplastic
Haematological
Infectious
Other
Scalp itch without skin lesions can be caused by neuropathic itch or could be psychogenic.
The lecturer then suggested a treatment suggestion for the scalp pruritus, starting with these first three steps:
And then continued with the medications that could relieve scalp pruritus:
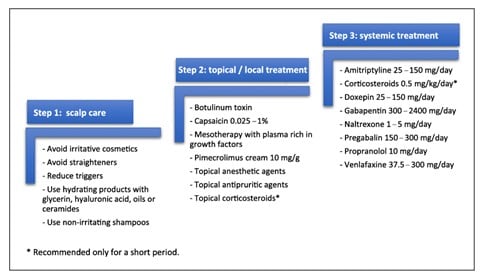
Figure 6. Review of treatments for scalp itch/sensitive scalp proposed in literature divided into three main pillars: scalp care, topical/local and systemic treatment (organized in alphabetical order).
Image source: Souza EN, Anzai A, Costa Fechine CO, Sakai Valente NY, Romiti R. Sensitive Scalp and Trichodynia: Epidemiology, Etiopathogenesis, Diagnosis, and Management. Skin Appendage Disord. 2023;9(6):407-415. doi:10.1159/000533795
Awareness of scalp pruritus is crucial for dermatologists, as it often precedes or accompanies underlying scalp disease and may serve as an early clinical clue to inflammatory or alopecias. If left untreated, chronic itch can significantly impair quality of life, disrupt sleep, and lead to secondary excoriations or lichenification. A thorough understanding of the multifactorial aetiology of scalp itch, along with targeted diagnostic evaluation and individualized treatment strategies, is essential for effective patient care and prevention of disease progression.
Literature:
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Lidia Rudnicka, Poland
Seborrheic dermatitis (SD) has a wide spectrum of clinical manifestations from mild scaling of the scalp to inflammatory lesions with scaling. Although previously disputable, all the experts at the EHRS meeting agreed that dandruff is part of the spectrum of SD. It is clinically presented as a non-inflammatory form of SD with signs of mild scaling.
Depending on the severity, the trichoscopic features of SD include:
A valuable advice is to start with dry trichoscopy since only then scaling would be visible. The immersion fluid gives insights into the vessels and inflammation of the scalp, including:
Scalp psoriasis usually affects 50-80% of the patients with psoriasis, and in approximately 25% of them, it is the first location of the plaque. It is often associated with the severe forms of psoriasis. Hallmark trichoscopic features and signs of the disease affecting the skin of the scalp include:
When differentiating between SD and scalp psoriasis, dermatologists should have this table in mind:
| Scalp Psoriasis | Seborrheic Dermatitis |
| White scaling | Yellowish scaling |
| Pink to red background | Normal to pink background |
| Linear arrangement of clusters of dotted vessels | Thin arborizing vessels |
| Dotted extravasations |
There are currently no official guidelines for the treatment of scalp psoriasis, since the European consensus is from 2009. However, advice on the therapy includes:
From the presented research analysis, it has suggested that interleukin-17 inhibitors seem to work most effectively in severe scalp psoriasis.
Prof. Rudnicka also explained the phenomenon of psoriatic alopecia, which should not be forgotten when treating scalp psoriasis. Psoriatic alopecia has been described as an associated side effect of the treatment of severe psoriasis with tumour necrosis factor (TNF) inhibitors (observed in rare cases after 2 – 46 months of treatment). This type of hair loss presents with a bald patch associated with severe pustular or plaque psoriasis. The alopecia can be both non-cicatricial (70%) or cicatricial (30%). The suggested hypothesis is that the perifollicular inflammatory infiltrates and the progressive sebaceous gland atrophy gradually led to hair follicle involvement and first non-cicatricial and then cicatricial alopecia.
On trichoscopy psoriatic alopecia presents with variable trichoscopy features of coexistence of psoriasis with features of anagen effluvium, telogen effluvium and cicatricial alopecia; clusters of vessels in linear arrangement and areas of no follicular openings (dots). The suggested treatment is the change of the biological medication.
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speaker: Rodrigo Pirmez, Brazil
Lichen planopilaris (LPP) is a chronic inflammatory condition that primarily affects the scalp, leading to cicatricial alopecia. Because LPP is characterized by inflammation around hair follicles, all the trichoscopic features will be therefore found around the hair follicle.
The trichoscopy features include:
It is important to remember the rare form of LPP with features of androgenic alopecia. The observation of perifollicular scaling when examining a patient with possible AGA is a red flag and should lead to a biopsy of the scalp to rule out LPP.

Figure 7. Lichen plano-pilaris management according to Pirmez. Blue: first line treatment; light blue: local treatments in association with oral treatment: first DC1 then in relay Tacrolimus; yellow: supportive treatment by oral minoxidil at any stage; green: second line; orange: third line and grey: other therapies
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speaker: Daniel Asz Sigall (Mexico) + from the posters by Stelmaszek et al. and Soplinska et al.
Pressure alopecia (PA) is a subtype of cicatricial and non-cicatricial alopecia occurring after pressure-induced obstruction of capillaries. Hypoxia of the hair bulb leads to circumscribed areas of hair loss. Two types have been described: type 1 (external compression) and type 2 (internal compression after the injection of any liquid during a cosmetic procedure). Type 1 presents as alopecic patches on bony prominences, while type 2 will occur in the vicinity of the injection area such as scalp, temporal area, forehead, or beard.
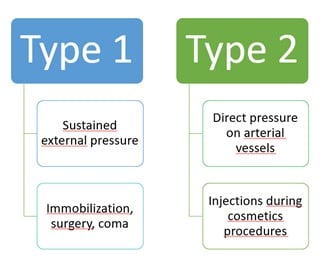
Clinical symptoms include during the later erythema, oedema, pain, ulceration, necrosis and then an irregular patch of alopecia. Trichoscopy is non-specific and may mimic alopecia areata, lichen plano-pilaris or trichotillomania. The prognosis is usually excellent; but in some case alopecia may be definitive. Stelamaszek et al. reported the case of a 30-yo-woman who had a cicatricial patch almost since birth as she stayed in an incubator for a long time due to prematurity.
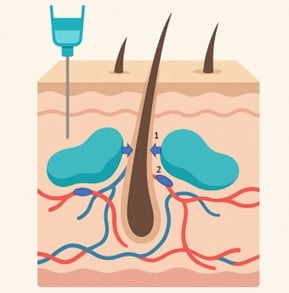
Figure 8. Physiopathology of pressure alopecia type 2 after cosmetic injection (fillers…). 1. Mechanical Pressure 2. Vascular thrombosis
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Adriana Rakowska
Postpartum alopecia, also known as postpartum telogen effluvium, is a self-limited form of diffuse hair shedding that commonly occurs 2 to 4 months after childbirth. It results from a synchronized shift of hair follicles from the anagen (growth) phase into the telogen (resting) phase, driven by the abrupt hormonal changes following delivery—primarily the decline in oestrogen levels. While physiologically normal, the sudden and often dramatic hair loss can cause significant emotional distress.
Postpartum hair shedding pathophysiological mechanism lies in delayed anagen release and hormonal changes during pregnancy. Delayed anagen release is a hair cycle disturbance in which a prolonged anagen phase is followed by a synchronized and premature shift of a large number of hair follicles into the telogen phase. This results in sudden, diffuse hair shedding usually up to 2-4 months after giving birth. The phenomenon reflects a disruption in the normal asynchronous cycling of hair follicles, and the shedding is typically self-limiting as follicles gradually re-enter the anagen phase.
Hormonal changes during pregnancy include very high amount of hormones: 9 times more progesterone, 4 times more estrone, 8 times more oestradiol, 9 times more estriol and 20 times more prolactin. The real “villains” responsible for hair shedding are progesterone and prolactin. Progesterone is responsible for the elongation of the anagen phase, increased hair shaft diameter and inhibition of androgen secretion. Therefore, during the second and third trimester more scalp follicles are in anagen phase. After delivery however, progesterone dramatically drops and induces catagen phase, while the prolactin levels remain high. Prolactin in turn is responsible for catagen and apoptosis induction. It has been estimated that prolactin levels go back to normal after 180 days after delivery and 7 days after breastfeeding. Therefore, after these days the hair follicles will most likely enter telogen simultaneously, with peak hair shedding at 3 to 6 months post-partum. After this period, hair shedding tends to normalize, and the hair regrowth is evident with 0.3-0.5mm daily growth.
Dermatologists are often asked whether it is possible to reduce postpartum hair loss by an early termination of breastfeeding for those women who suffer from this condition. Unfortunately, studies show that hormone replacement therapy was not effective for post-partum hair shedding.
And could postpartum telogen effluvium (PPTE) induce female pattern hair loss (FPHL)? Unfortunately, yes. 75-84% of the patients with PPTE have FPHL, and in fact PPTE is more severe in those women. Pregnancies may stimulate FPHL in genetically predisposed females as well.
In addition, dermatologists should be also aware of post-partum iron deficiency, which may trigger more telogen effluvium, and also postpartum thyroiditis which occurs in 5% of the new mothers.
Rakowska then suggested a therapeutic approach:
Low dose oral minoxidil and spironolactone may be also considered; but finasteride, dutasteride or bicalutamide should not be suggested.
Postpartum alopecia has a typically benign and reversible nature but should be differentiated from other forms of hair loss such as androgenetic alopecia or telogen effluvium of other aetiologies. Proper diagnosis, patient education, and supportive management —including addressing nutritional status, scalp health, and hair care practices— are essential to reduce anxiety and avoid unnecessary interventions. In some cases, persistent shedding may unmask underlying chronic hair disorders, underscoring the need for careful follow-up.
Literature: Gizlenti S, Ekmekci TR. The changes in the hair cycle during gestation and the post-partum period. J Eur Acad Dermatol Venereol. 2014;28(7):878-881. doi:10.1111/jdv.12188
Report written by Dr Lidiya Todorova (Dermatologist, Bulgaria)
Speaker: Andy Goren, Italy
Minoxidil, originally developed as an antihypertensive agent, has become a cornerstone in the management of various hair loss disorders. Topical minoxidil is the only FDA approved drug for the treatment of both male and female androgenetic alopecia (AGA). It promotes hair growth by prolonging the anagen phase, enhancing follicular size, and improving scalp blood flow. In recent years, low-dose oral minoxidil (LDOM) has emerged as a promising off-label alternative, especially in patients who experience scalp irritation or suboptimal response to topical formulations.
Clinical studies show that response to 5% topical minoxidil is typically observed after 16 to 24 weeks of treatment. They also demonstrate that following the 4 weeks of treatment there is approximately 30-40% responders, i.e. subjects with hair regrowth1.
While the exact mechanism of action of minoxidil in the treatment of female pattern hair loss (FPHL) is not completely understood, research has demonstrated that minoxidil sulfate is the active compound that stimulates hair follicles2. Minoxidil is a pro-drug and is converted to its active form, minoxidil sulfate, in the outer root sheath of the hair follicle by endogenous sulfotransferase enzymes – primarily SULT1A12,3.
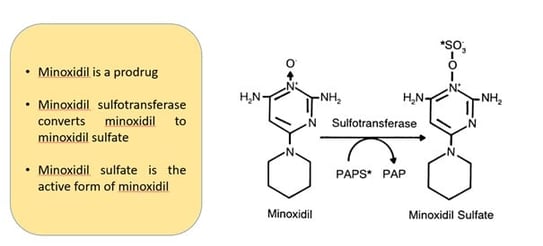
Figure 9. Minoxidil mechanism of action: Sulfation of minoxidil catalysed by sulfotransferase. PAPS* represents [35S]-3%-phosphoadenosine5%-phosphosulfate and PAP represents 3%-phosphoadenosine-5%-phosphate.
Image Source: Anderson, R. J., Kudlacek, P. E., & Clemens, D. L. (1998). Sulfation of minoxidil by multiple human cytosolic sulfotransferases. Chemico-Biological Interactions, 109(1-3), 53–67. doi:10.1016/s0009-2797(97)00120-8
Due to the significant time commitment and low response rate, a biomarker for predicting patient response prior to therapy would be advantageous. Sulfotransferase activity in the hair follicle as a strong predictor of minoxidil response in AGA patients. SULT1A1 enzyme activity assay demonstrates 95% sensitivity and 73% specificity in predicting minoxidil treatment response for AGA4.
Nevertheless, Goren the suggested that according to the studies there are about 60-70% of the AGA patients that do not respond to topical minoxidil and posed the question about their treatment.
SULT1A1 enzyme is a cytosolic phase II metabolizing enzyme, which is highly expressed in the liver ad to lesser extent in the skin and scalp. The primary function of the enzyme is elimination of xenobiotics. The enzyme sulfonates phenol substances thus increasing their solubility and subsequent elimination from cells. SULT1A1 expression is positively correlated with the expression of sulfate efflux transporter ABCC3 as increased sulfonating capacity requires adequate efflux transport of the sulfonated moieties. Topical minoxidil in other hand is a small molecule, which easily enters keratinocytes and is subsequently sulfonated inside keratinocytes and excreted via the ABCC3 efflux transporter.
In contrast, oral minoxidil is primary sulfonated by the SULT1A1 enzyme in the liver, thus resulting in 80% of the oral dose eliminated in the urine within 30 minutes of ingestion. Therefore, a novel unknown pathway is responsible for the hair regrowth effect of oral minoxidil. Goren then presented a study involving 41 patients, 26 males and 15 females), receiving 5mg and 1.25mg of oral minoxidil, respectively to the gender. Following 6 months of dosing, clinical improvement was observed in 26 patients, and the response was higher in men (19/26) than in women (6/15). Optical density of the hair was achieved by 85% of the subjects.
Studies also suggest that minoxidil sulfate cannot enter cells without active transport by the SCL22A9 influx transporter. Cells expressing high levels of SULT1A1 and the corresponding sulfate efflux transporter ABCC3 tend to express low levels of the SCL22A9 sulfate influx transporter. This makes sense biologically as cells producing high amounts of sulfonated moieties need to eliminate them rather than absorb additional sulfates. However, in cells with low sulfate activity, there is a need for improving sulfate and thus higher expression of the sulfate influx transported SCL22A9. There are variations in the expression of the drug transporters in the kidney when comparing gender. This influences pharmacokinetics of minoxidil sulfate, as the SCL22A9 sulfate influx transporter has a higher expression in males versus females. This may explain why 40% of women and 73% of men responded to LDOM for the treatment of AGA.
When investigating the impact of environmental factors, it has been demonstrated that the hypoxia upregulates SCL2A9 expression by downregulating ABCC3 genes. The regulatory changes in ABC transporter genes in response to a hypoxic environment may contribute to the substantial increase in SCL2A9 expression. In fact, the expression of SCL2A9 exhibited a 1.90-fold change increase within breast cancer cells exposed to hypoxia when compared to those under normoxic conditions. Acetaminophen hepatotoxicity is primarily attributed to sulfonation. To counteract this toxicity, deferiprone (deferoxamine) is frequently employed. Notably, deferoxamine, an inducer of HIF-alpha, has been demonstrated to upregulate SCL22A9. Rifampicin, which has been found to suppress SCL22A9, has been associated with an increase in acetaminophen sulfate plasma concentration and the potential of hepatotoxicity. Therefore, the activity of the SULT1A1 enzyme in plucked hair follicles serves as a marker of predicting LDOM response for the treatment of AGA. In contrast to topical minoxidil, low SULT1A1 enzyme activity in hair follicles predicts response of the oral formulation. HIF pathway may also prove a mechanism to increase drug response to minoxidil.
It is increasingly important for dermatologists to be aware of both topical and oral minoxidil formulations, as they offer flexible, well-tolerated, and often synergistic treatment options for non-scarring alopecias. The growing body of evidence supporting the efficacy and safety of LDOM—at doses as low as 0.25–2.5 mg daily—has expanded its use, particularly in difficult-to-treat cases and in patients preferring oral therapy. Understanding the indications, dosing, side-effect profiles, and patient selection criteria for both routes of administration is essential for optimizing hair restoration outcomes in the dermatologic practice.
Literature:
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speakers: Sergio Vañó-Galván (Spain) & Yuliya S. Ovcharenko (Ukraine)
Regarding breast cancer, two recent studies – one databased study and one retrospective cohort study – showed that oral 5-alpha reductase inhibitors were not associated with either breast cancer or benign breast tumours. Those results highlight potential off label use for female pattern hair loss with minimal cancer risk concerns.
In Spain, the current consensus from the Spanish Trichology Group of the AEDV for managing female androgenic alopecia include:
Low-dose oral minoxidil (0.5–1 mg/day) or topical 5%, combined with spironolactone (100–200 mg/day) or 5-alpha-reductase inhibitors (finasteride or dutasteride).
In 2012, Post-Finasteride Syndrome (PFS) emerged as a controversial condition reported by some patients after discontinuing finasteride, for male pattern baldness. Symptoms included initially sexual dysfunction (erectile dysfunction, low libido, …). As PFS was widely publicized, pharmacovigilance reports of finasteride-associated mental health issues (depression, anxiety) and suicide increased. PFS is characterized by sexual dysfunction, somatic symptoms, and psychological disorders that persist after cessation of finasteride treatment.
The hypothesis would be that the inhibition of the 5α-reductase enzyme by finasteride results in lower levels of allopregnanolone, which has antidepressant and anxiolytic effects.
VigiBase (WHO's global database of adverse event reports for medicines and vaccines) analysis revealed 356 reports of suicide and 2926 reports of psychological adverse events in finasteride users, 70.9% with data available aged 18-44 years. A significant disproportionality signal for suicide (ROR, 1.63; 95%CI, 1.47-1.81 for suicidal ideation and psychological adverse events (ROR, 4.33; 95%CI, 4.17-4.49) in finasteride was identified. It is important to stress that health professionals including clinicians, pharmacists, and nurses, and patients can report suspected adverse drug reactions in Vigibase.
In sensitivity analyses, younger patients with androgenetic alopecia (AGA) had significant disproportionality signals for increased suicidality, while such signals were not detected in older patients with benign prostatic hyperplasia. Sensitivity analyses also showed that the reports of these adverse events significantly increased after 2012, when reports started emerging on men who had used finasteride and either attempted or completed suicide.
Besides, it is important to stress the lack of suicide signal observed for dutasteride, a drug that is like finasteride in the mechanism of action but has not attracted as much media attention, and the absence of a dose-response association with finasteride, suggests a potential reporting bias unique to finasteride.
A systematic review and meta-analysis of 5 studies that included 2,213,600 patients (228,453 users of 5-alpha reductase inhibitors vs. 1,985,147 controls) found no significant association was found between 5-ARI use and the risk of depression or suicide, including at the commonly prescribed 1 mg/day dose for alopecia.
However, the EMA's Pharmacovigilance Risk Assessment Committee has recently stated that suicidal ideation is a side effect of finasteride tablets in patients using the 1 mg dosage for androgenetic alopecia, although the frequency is unknown.
Despite these risks, the benefits of finasteride and dutasteride continue to outweigh their risks for approved uses.
Of note, some hair specialists recommend appropriate to ascertain a personal history or screen for preexisting mental health issues for a stringent selection of patients before starting them on finasteride, since these may put patients at an increased risk of developing emotional disorders, such as depression, and somatization, such as the PFS (Trüeb RM, Gavazzoni Dias MFR, Dutra Rezende H. Suicidality and Psychological Adverse Events in Patients Treated with Finasteride. Skin Appendage Disord. 2021 Nov;7(6):524-526)

Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speaker: Rodrigo Pirmez, Brazil
There are currently not many publications regarding the management of hair loss in transgender (TG) individuals. As a reminder a transgender male (TGm) is a person who was assigned female at birth but identifies as a man. TGm may choose to undergo gender-affirming hormone therapy (GAHT; testosterone) and/or gender-affirming surgeries. A transgender female (TGf) is a person who was assigned male at birth but identifies as a woman. GAHT includes oestrogen and anti-androgens. TGf may undergo surgeries to feminise their appearance.
In the orator’s experience, androgenic alopecia (AGA) represents the 6th cause of consultation in his clinic in Brazil, 7.5% of the consultation behind acne (29%), cosmetic procedures (27.1%), cysts (11.2%), pseudofolliculitis barbae (10.3%) and acne scars (9.3%)
AGA develops one to 5 years after GAHT (testosterone initiation), although hair modifications can be seen as early as 6 months (diminution of hair width). AGA affects 17% of TGm without GAHT and 43 to 60% with GAHT. Family history of AGA and duration of GAHT are the main risk factors for AGA.
Treatment includes minoxidil 2.5-5 mg/daily as for cis gender males. The higher dosage is well accepted as it will also increase the body hair counts. Finasteride 1 mg/day can be associated with minoxidil. However, it is recommended to start finasteride only one to two years after GAHT initiation. First because AGA is of late onset and second because there are questions regarding the impact of early initiation of a 5 alpha reductase inhibitor on the development of facial/hair body and clitoral enlargement. The place of dutasteride is not known (in case of failure of finasteride?). Spironolactone is contra indicated in TGm. Lastly, non-operated TGm should need contraception as testosterone does not prevent pregnancy.
Regarding beard growth in TGm, facial hairs start to develop as early as 3 months. However, there are discrepancies between individuals and not everyone has a full-grown beard. Topical or oral minoxidil can be used. Finally, facial hair transplants are possible.
GAHT (oestrogens and antiandrogens) can halt the progression of AGA in TGf and can even lead to reversal of AGA. Management of AGA in TGf improves gender dysphoria and quality of life. If necessary, spironolactone 50-200 mg/d associated with oral minoxidil can be used. Oral minoxidil should be kept at a low dose like 0.5 mg/daily to avoid gender dysphoria (due to increase of body hairs). Surgery such as hair transplant or scalp advancement can be provided as well.
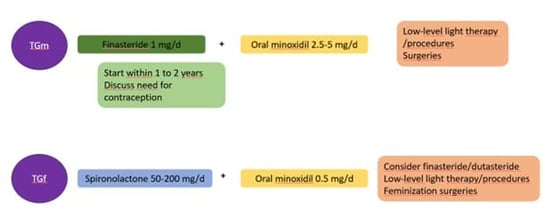
Figure 10. Summary of the management of AGA in transgender individuals according to Pirmez (TGf: Transgender female; TGm: Transgender male)
Report written by Dr Nicolas Kluger (Dermatologist, Finland)
Speakr: Arash Mostaghimi, USA
Pr Mostaghimi presented a study published in JAMA Dermatology 2021 that evaluated the prevalence and magnitude of laypersons’ stigma toward individuals with varying degrees of alopecia and whether stigma increases with increased severity of alopecia (Creadore A, Manjaly P, Li SJ, Tkachenko E, Zhou G, Joyce C, Huang KP, Mostaghimi A. Evaluation of Stigma Toward Individuals with Alopecia. JAMA Dermatol. 2021 Apr 1;157(4):392-398, accessible for free https://pmc.ncbi.nlm.nih.gov/articles/PMC7948115/)
The authors generated AI and stock images portrait images of 6 individuals without hair loss. Each portrait was edited to create 2 additional versions, 1 with scalp hair loss and 1 with complete hair loss, for a total of 18 images. An internet survey was completed by 2015 respondents. The survey included a series of stigma-related questions from 3 domains: stereotypes, social distance, and disease-related myths (if the respondents believed that the individual pictured had a medical condition).
As the severity of alopecia increased, more respondents endorsed each stereotype and social distance item. The most significant increases in stereotype endorsement were for "sick" (a 27.6% rise) and "unattractive" (a 16.5% rise) in case of complete hair loss. Regarding social distance, the largest changes were an 18.3% increase in disagreement with the statement “I would find the person in this photo attractive” and a 6.9% increase in disagreement with “I wouldn’t mind having physical contact with the person in this photo.”
The degree of increase in stigma from the original version to the complete hair loss version depended on the skin tone and sex of the individual pictured. Increasing alopecia severity across all portrait categories was associated with an increased belief among respondents that the individual pictured had a medical condition. These findings suggest that laypersons may stigmatise individuals with alopecia, that their stigma increases with alopecia severity, and that it can be influenced by the patient’s race and sex.

Figure 11. Sample of computer-generated portraits and 2 versions with varying degrees of alopecia
Image source: Creadore A, Manjaly P, Li SJ, Tkachenko E, Zhou G, Joyce C, Huang KP, Mostaghimi A. Evaluation of Stigma Toward Individuals With Alopecia. JAMA Dermatol. 2021 Apr 1;157(4):392-398)