0 professionals
CARD 2024 Bioderma Congress Reports
CARD 2024 Bioderma Congress Reports
Get access to exclusive dermatological services to increase your professionnal knowledge: +500 pathology visuals, clinical cases, expert videos
Benefit from valuable features: audio listening, materials to be shared with your patients
Stay informed about the upcoming events and webinars, latest scientific publications and product innovations
Already have an account? login now
This year we're celebrating 40 years of CARD! This adventure began in Lyon in 1982 with the creation of the Société de Recherche en Dermatologie (SRD), focused on fundamental research, by Professor Jean Thivolet, and the creation of the Centre de Biologie de la Peau (Skin Biology Centre) in 1983 by Daniel Schmitt. The first congress was held in Toulouse in 1983.
In 1992, the first Human Skin and Society Forum was held, and in 1997, the European Journal of Dermatology was launched with Jean-François Nicolas.
Related topics
Moderators: Fabien Chevalier & Soline Estrach
Soline Estrach, IRCAN, Nice
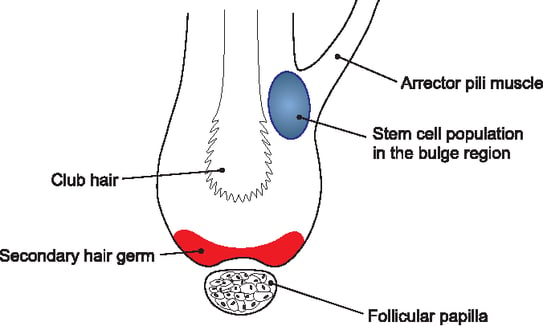
The growth and maintenance of the hair follicle takes place in a three-phase cycle:
Andrei A. Panteleyev, Colin A. B. Jahoda, Angela M. Christiano; Hair follicle predetermination. J Cell Sci 1 October 2001; 114 (19): 3419–3431. doi: https://doi.org/10.1242/jcs.114.19.3419
The initial activation signal (via the Wnt signalling pathway) comes from the dermal papilla (follicular papilla). However, other stem cell activation signals emanate from the hair follicle niche(bulge). The hypothesis is that epithelial stem cells produce their own niche, enabling the structure of the hair follicle to be maintained.
To study the signals leading to the formation and maintenance of this niche, mice transgenic for a reporter gene were used. The GFP (Green Fluorescent Protein) gene was inserted after the Lrig1 gene (marker for hair follicle epidermal stem cells). Using fluorescence microscopy, stem cells expressing Lrig1 are visible in green within the hair follicle and can be sorted by flow cytometry to compare them with basal keratinocytes.
Comparison of the gene expression profile of epidermal stem cells compared with basal keratinocytes shows that the signalling pathways over-expressed in stem cells involve integrins (Int), and all the signals downstream of the extracellular matrix (matrisome), including fibronectin.
Fluorescence microscopy analysis of back skin and tail sections of mice shows the formation of a fibronectin network specific to the stem cell activation phase. In mouse embryos in which a deletion of the fibronectin gene is induced before endogenous activation of hair growth, there is a total absence of hair growth with a notable thinning of the hair afterwards (similar to the phenotype observed during ageing). When the deletion is performed later in the mouse's development, the same results are obtained. In addition, hair regrowth was observed in mice deficient in the fibronectin gene but transfected exogenously with this gene.
In the absence of fibronectin, a large proportion of Lrig1-labelled cells are located in the infundibulum of the hair (instead of migrating to the hair matrix at the base of the hair). However, the proliferation marker Ki67 was not increased in these cells. Therefore, it is not an increase in cell proliferation but a migration process that takes place during the regeneration process.
In summary, fibronectin expression by epithelial stem cells is necessary for follicle regeneration, localisation of hair follicle stem cells (Lrig1 positive) within follicles and maintenance of this population.
To study the signalling cascade induced by fibronectin, a mouse model KO for the SLC3A coreceptor was developed. It is impossible to directly delete the integrin gene without risking the loss of keratinocyte cohesion. In the absence of SLC3A, there was no difference in fibronectin expression in the total skin of mice, but a defect in fibronectin network assembly was observed. In this model, Lrig1 cells are found delocalised in the infundibulum, as previously observed in fibronectin KO mice, confirming the specific role of fibronectin in the localisation of hair follicle stem cells.
The creation of a fibronectin migration pathway enables the regeneration signal to be induced via an integrin-mediated mechanotransduction signal. In the absence of SLC3A, this mechanotransduction signal is lost. Activation of the Int/SLC3A2/mechanotransduction cascade is involved in the regenerative potential of stem cells.
In conclusion, stem cells must be able to assemble fibronectin to induce proliferation signalling and maintain an undifferentiated state without entering a differentiation pathway.
Chloé Chave, Lyon
The mechanical microenvironment plays a crucial role in keratinocyte homeostasis. It has been shown that on a rigid support, keratinocytes adopt proliferative behaviour, whereas on a soft support they tend to differentiate while retaining their ability to proliferate.
Synaptopodin is a protein expressed in the neurons and podocytes of the kidneys. Retinoic acid increases the expression of this protein. Fluorescence microscopy and RNA analyses show that it is also expressed in skin cells, particularly in the basal layer of the epidermis. The aim of the study presented in this oral communication is to investigate the role of this protein in keratinocyte homeostasis.
Synaptopodin expression has been shown to decrease during keratinocyte differentiation. Keratinocytes KO for synaptopodin continue to proliferate normally, but wounding tests show a reduction in keratinocyte migration in the absence of synaptopodin. The literature indicates that synaptopodin is bound to the cytoskeleton, in particular actin filaments. In western blots with immunoprecipitation, synaptopodin and keratin 14 co-precipitate, demonstrating an interaction between the two proteins. This result is supported by the colocalisation of these two proteins in the cell using fluorescence microscopy.
Keratin 14 is known to be involved in cell adhesion. Synaptopodin could therefore be a negative regulator of keratin 14. Thus, in the context of a rigid microenvironment, there would be polymerisation of keratin 14 and rigidification of the membrane, with retinoic acid capable of inducing the expression of synaptopodin, exerting negative feedback on the polymerisation of keratin 14. Therefore, Synaptopodin would limit the cell rigidity promoted by keratin 14 and maintain the cell's proliferative homeostasis without inducing differentiation.
Laure Gibot, SOFTMAT, Toulouse
Oxidative stress generates radical species characterised by a single electron on the atom's peripheral layer. These unstable and potentially dangerous species are of two main types: reactive oxygen species (ROS) and reactive nitrogen species (RNS). Oxidising species are known to induce oxidative damage within cells, but paradoxically they also play a crucial role in cell signalling. Under physiological conditions, the redox balance is maintained. In the event of oxidative stress, an imbalance occurs, either through increased production of oxidising species or a shortage of antioxidants.
Laure Gibot presented a reconstructed human epidermis model used to study the effects of oxidative stress. The cells produce their own matrix in the presence of vitamin C after four weeks, creating a dermal substitute that can be manipulated under different conditions.
The first stress studied was induced by pulsed electric fields, causing electroporation and defects in cell membranes. This system, already used to vector molecules within cells (for example, electrochemotherapy for skin cancers), also enables cell fusion and, in some cases, induces cell death. After application of the electric field, a decrease in TGF-β production was observed, accompanied by a decrease in procollagen I and collagen I synthesis in the medium at 24 hours. This change was accompanied by a reduction in the expression of lysyl oxidase and transglutaminase 2, which are involved in the maturation of the extracellular matrix. Conversely, MMP (matrix metalloproteinase) activity increases up to at least 48 hours after electroporation. In the presence of MMP inhibitors, collagen production is normalised. It has been shown that ROS are responsible for reducing the synthesis of the extracellular matrix. Electroporation therefore appears to generate oxidative stress which remodels the extracellular matrix by increasing MMP activity, leading to collagen degradation and a reduction in collagen synthesis. Physical stress therefore influences the synthesis, maturation and degradation of the matrix via the generation of ROS. However, the exact nature of these ROS and the mechanism by which they are formed remain to be elucidated.
This action is reversible and could represent an antifibrotic therapeutic approach, or promote the delivery of anti-cancer molecules.
Another stress model presented by Laure Gibot is photodynamic therapy (PDT). PDT is based on the use of a photosensitiser which, under the action of a certain wavelength, generates ROS. This method has a variety of applications, such as anti-cancer or anti-bacterial treatments, or as a rejuvenation method (filling in wrinkles). Photosensitisers - often hydrophobic aromatic molecules - form aggregates that are deleterious to the generation of ROS. The use of micelles avoids these aggregates and facilitates the integration of the photosensitisers into the cells.
In this study, pheophorbide (Pheo) is used as a photosensitiser. By applying it to dermal substitutes, the researchers demonstrated good penetration of the encapsulated micellar form, unlike the free form. The aim is to determine the concentrations of free or encapsulated Pheo needed to avoid cytotoxicity and obtain a sublethal effect, generating ROS.
Transcriptomic analysis revealed that free Pheo did not affect cell gene expression. Conversely, encapsulated Pheo induces major changes in transcripts, mainly genes involved in oxidative stress response pathways.
PDT can therefore be used to modulate oxidative stress locally, inducing an interesting sublethal antioxidant response in cells in the face of this stress.
Moderators: Marion Salou & Marc Vocanson
Marion Salou, Institut Curie, Paris
MAIT (Mucosal Associated Invariant T cells) are T lymphocytes found in the mucous membranes (skin, lungs, intestine) as well as in the spleen and liver. The TCR (T cell receptor) of MAITs has the particular feature of being semi-invariant (an invariant TCRα chain associated with a limited number of TCRβ chains), recognising small bacterial antigens, corresponding to metabolites of the riboflavin synthesis pathway. These immune cells possess a memory phenotype allowing them to integrate TCR and cytokine signals, and respond accordingly. This phenotype is acquired during maturation in the thymus, where they undergo selection via epithelial thymic cells.
In addition to their antibacterial properties, MAITs express a transcriptomic program of tissue repair and promote the healing of skin wounds. The study presented by Marion Salou aimed to highlight a possible role for MAITs in the healing and tissue repair process. To do this, a mouse model expressing more MAIT than wild mice was used to get closer to human conditions. The results show that after an injury, the number of MAITs in the injured area is multiplied by ten. In contrast, in MAIT-deficient mice, tissue repair time is prolonged, highlighting the potential role of MAITs in wound healing. This accumulation of MAITs at the wound site is not due to their proliferation, as the Ki67 proliferation marker is not increased, suggesting increased recruitment of MAITs. Transcriptomic data indicate a likely involvement of CXCR6 receptors and, to a lesser extent, CCR2 receptors.
To verify the involvement of CXCR6, a deletion of CXCR6 was carried out in a cell culture enriched in MAITs, confirming that the recruitment of MAITs was no longer effective in the absence of CXCR6. The MAITs seem to promote tissue repair by promoting the proliferation of keratinocytes, as evidenced by the increase in Ki67 within the keratinocytes. To determine the mechanisms by which MAITs induce this proliferation, MAITs were extracted from the thymus and skin in the basal state and after injury, in order to compare gene expression by the Single Cell RNA Seq method. In the MAITs found after an injury, an increase in the cluster of "repair" genes was observed compared to the MAITs in the basal state. Among this cluster, two molecules are particularly involved: amphiregulin and IL-8. The involvement of amphiregulin in healing has been demonstrated in mice deficient in amphiregulin, whose healing time is significantly prolonged.
The literature shows that TCR can also play a role in the MAIT-mediated tissue repair process. However, MAITs adopt the same behaviour in vivo even when they have lost their TCR. This suggests that the expression of the repair function is probably independent of continuous TCR stimulation. It is also possible that TCR activation may have a synergistic effect with CXCR6 activation. Adding 5-OP-RU -a bacterial TCR ligand - to the wound increases the ability to repair.
In summary, MAITs are recruited from the blood and lymph nodes via the CXCR6 receptor at the injured tissue site. Their involvement in the healing process appears to be mediated by amphiregulin.
Lilian Basso, Infinity Institute, Toulouse
Nociceptors are sensory neurons whose cell bodies are located in the spinal ganglia along the spinal cord. Their axon divides into two branches, one towards the spinal cord and the other towards the periphery. These neurons are minimally or not at all myelinated, and are small and medium in size. They are bidirectional, driving nerve signals from the periphery to the central nervous system and participating in the inflammatory response by secreting neuropeptides at the periphery. They express specific sodium channels such as NaV 1.8. There are several populations of nociceptors, divided into three distinct groups: non-peptidergic neurons, peptidergic neurons and neurons expressing low threshold mechanical receptors.
The role of nociceptors is well documented in the context of atopic dermatitis. For example, the detection of microorganisms such as Staphylococcus aureus or mites leads to the activation of nociceptors via TRPV1, and to the release of substance P, responsible for the degranulation of mast cells via their MRGPRB2 receptors. This results in the recruitment of leukocytes from the bloodstream and the initiation of the Th2 response.
In contrast, the mechanisms that cause allergic contact dermatitis (ACD) differ. This pathology involves a Th1 and Th17 type inflammatory response and evolves in two phases. The first phase, called sensitisation, begins with the penetration of the hapten through the epithelial barrier. The hapten then binds to a self-antigen, forming a neo-antigen recognised by antigen-presenting cells, such as Langerhans cells. These cells then migrate to the lymph nodes to activate the lymphocytes, forming resident memory lymphocytes. During a rechallenge by the hapten already encountered, an inflammatory response characteristic of ACD is induced.
Lilian Basso presented a model of mice lacking NaV 1.8, thus inactivating the nociceptors. This model makes it possible to highlight a potential role of nociceptors in ACD. In these NaV 1.8 deficient mice, the inflammatory response is exacerbated, although the mice scratch less: this suggests a decoupling between the intensity of the inflammatory reaction and pruritus. This increased inflammatory reaction is characterised by a strong infiltration of polynuclear neutrophils.
In the spinal ganglia of mice, it is possible to isolate those specifically innervating the skin, thanks to a retrograde tracer, DiI, injected into the dermis of mice and going up to the ganglia. In this way, the specific transcriptomic data of these neurons innervating the skin are obtained. In inflammatory conditions, a new population of neurons appears, at a maximum on the fourth day of inflammation, with a characteristic transcriptomic signature. This population appears to form from the dedifferentiation of pre-existing non-peptidergic neurons. Over-expressed genes are those associated with neuronal regeneration following nerve injury, such as SOX11 and ATF3 (Activating Transcription Factor 3). ATF3 is a transcription factor that plays an important role in the cellular response to stress and injury. When expressed by neurons, ATF3 regulates the expression of genes involved in axonal growth and nerve tissue repair, as well as the inflammatory response. This response is reversible, because 60 days after the challenge inducing ACD in mice, ATF3 expression disappears. In mice where the ATF3 gene has been inactivated, a significant decrease in pruritus and infiltration of neutrophils is observed. On the contrary, in mice with a depletion of the TRPV1 channels, an increase in the inflammatory reaction is noted, decorrelated from the scratching behaviour.
In summary, several populations of neurons are involved in the ACD mechanism, including TRPV1 peptide neurons with a rather anti-inflammatory role and ATF3-producing non-peptidergic neurons involved in scratching behaviour.
Moderators: Judith Fischer & Jérôme Lamartine
Judith Fischer, University of Freiburg, Germany
Ichthyoses are a group of skin diseases characterised by extreme dryness and abnormal desquamation. The classification of ichthyoses is done at several levels. Distinctions are made between:
Ichthyoses can also be classified by their mode of transmission, with some forms being autosomal dominant or recessive , and others being X-linked. Thanks to the development of high-throughput sequencing, it is increasingly possible to isolate specific genes involved in the occurrence of ichthyoses.
In the case of ARCI, including lamellar ichthyosis, the mutations identified mainly concern enzymes involved in the metabolism of skin lipids. Judith Fischer described a newly identified mutation, NKPD1, causing dominant lamellar ichthyosis. Patients typically present with mild lamellar ichthyosis with fine scales on the trunk, arms, and head. Rather grey-coloured scales are present on the neck. Desquamation increases in high temperatures, in humid conditions and during sweating.
The NKPD1 gene codes for a KAP family NTPase protein with a “P-loop” domain. P-loop NTPases are enzymes involved in many cellular mechanisms, including mRNA translation, signal transduction, cell mobility, and cell growth. P-loop domains are characterised by two transmembrane domains conserved among all members of this family. Loss of either of these two domains results in loss of protein function. It is highly expressed in epithelial cells and, to a lesser extent, in central nerve cells and the thyroid. To date, there is no data in the literature regarding its role in keratinocyte differentiation, and its potential interactions with other proteins are not known. The Gene Network Predictions tool indicates that it would be involved in different pathways, including the formation of the stratum corneum of the epidermis and the metabolism of sphingolipids.
In biopsies from patients with this mutation, the stratum corneum is thicker with marked acanthosis. In immunohistochemistry, the protein is slightly more expressed in keratinocytes of the basal and granular layers, but much less expressed in melanocytes. Analysis of skin ceramides from patients with this mutation, performed by scotch test, does not show any significant change compared to healthy individuals (3 patients), but there is an increase in protein-bound ceramides. These studies are preliminary for the moment.
In conclusion, NKPD1 is a genotypic variant with very low prevalence. However, given the very mild skin damage, it is likely that patients will not seek medical attention for this reason. There are currently more than 100 known forms of ichthyosis. A large proportion of the genes involved in the disease are part of lipid metabolism. The gene mutations that cause about 10% of ichthyoses are not yet known.
Lea Pechtimaldjian, Bordeaux
Telocytes are cells known to provide vascular remodelling by preventing the anarchic development of vessels. The aim of the study presented by Léa Pechtimaldjian was to evaluate their role in benign childhood tumours, such as haemangiomas. The work was conducted on human tissues 400 µm thick, requiring them to be rendered transparent in order to be studied by light microscopy. Three-dimensional vascular labelling was performed to obtain 3D image reconstruction.
There is a phenomenon of vascular normalisation in the presence of telocytes within haemangiomas. In proliferative haemangiomas, telocytes are organised in a sheath around the vessels, unlike in involuting haemangiomas where telocytes form a looser mesh.
One method used for endothelial cell culture uses collagen beads embedded in a fibrin hydrogel. Thus, cells can bud within the gel and reconstitute vascularisation in vitro. By co-culturing endothelial cells and telocytes, an anti-angiogenic effect is shown via the secretion of factors into the medium. This effect is specific to haemangioma telocytes, as normal telocytes have no impact on vascularisation.
The next step will be to characterise the anti-angiogenic factors in order to develop a tool to combat tumour neoangiogenesis.
Julien Ablain, CRCL, Lyon
Currently, the treatment of melanoma presents three major challenges:
Over-expression of the MAP kinase (MAPK) pathway in melanocytes is the main mechanism behind melanomas, particularly with gains-of-function of the BRAF, NRAS or KIT genes. At the same time, the inactivation of certain tumour suppressor genes acts synergistically in the process of carcinogenesis. The CDKN2A gene is mutated in 40 to 60% of melanoma cases. Among all cancers, melanoma has the highest number of mutations per portion of the genome. It is therefore complex to highlight all the driver mutations, hence the importance of developing genetic models in order to study the role of these different mutations. Julien Ablain presented a vector system for modelling the genetics of human melanoma in zebrafish.
This system, called MAZERATI, is based on the combination of two vectors allowing the over-expression of an oncogene and another gene of choice. These vectors are integrated into the zebrafish embryo and stably insert into the adult genome. The appearance of tumours in adult fish can thus be observed. Spontaneous tumours with a genotype similar to that of human melanoma tumours can be generated, for example, by over-expressing BRAF V600E and inactivating a tumour suppressor gene such as PTEN or P53. With these tools, it is thus possible to conduct human genetic studies in fish and to investigate gene alterations within tumours.
Here we focus on the SPRED1 gene (Sprouty Related EVH1 Domain Containing 1), known to be a negative regulator of the MAPK pathway. A mutation in this gene causes Legius syndrome, which resembles neurofibromatosis type 1. SPRED1 inhibits the MAPK pathway, possibly by directly interacting with NF1 and promoting RAS inactivation. The SPRED1 mutation is particularly found in the presence of a prior KIT alteration. Using the model described above, it is possible to form melanocytic tumours with KIT mutation, over-activating the MAPK pathway and inactivating SPRED1. It is shown that, in this context, the generation of melanomas occurs more rapidly than with the KIT mutation alone. Selection pressure appears to be exerted on cells mutated for SPRED1 when KIT is already mutated. Loss of SPRED1 induces resistance to targeted therapies, such as the KIT inhibitor Dasatinib, highlighting the importance of knowing about the existence of this mutation.
Another deletion frequently found in melanoma tumours is the mutation of the Nectin 1 gene. Nectin 1 is a cell surface adhesion molecule forming early adherens junctions, even before the involvement of cadherins. A lower level of Nectin 1 expression was demonstrated in metastases compared to primary tumours. Using the model described above, tumours in which Nectin 1 has been inactivated metastasise more frequently. The loss of Nectin 1 therefore appears to promote the dissemination of melanocyte cells. This hypothesis is tested by injecting Nectin 1 KO tumour cells into healthy fish, where the tumour cells disseminate more than the melanoma cells expressing Nectin 1.
In cell culture, migration of Nectin 1 KO cells is increased only in the absence of serum. In fact, under normal conditions, melanocytes do not form adherens junctions, because they are not epithelial cells. In the absence of serum, melanocytes undergo stress which leads them to form adherens junctions. Nectin 1 KO cells are unable to form these junctions in the absence of serum, and adopt a morphology more suited to migration. It appears that IGF1 and insulin (present in the serum of cell cultures) prevent the formation of adherens junctions. Thus, in the presence of IGF1, the presence or absence of Nectin 1 has no impact since the cells do not need to form adherens junctions. On the other hand, in the absence of IGF1, the presence of Nectin 1 is necessary to form adherens junctions, the cells being subjected to stress. When cells are KO for Nectin 1, the loss of adherens junctions leads to cell migration.
Moderators: Vincent Flacher & Bérengère Fromy
Vincent Flacher, Institut de Biologie Moléculaire et Cellulaire (IBMC), Strasbourg
We now know that the skin's immune cells are not the only ones responsible for mediating the inflammatory response in inflammatory skin diseases. The microenvironment plays a crucial role in the activation of Langerhans cells and the polarisation of the T-cell response, with neurons playing a major role. For example, in atopic dermatitis, numerous studies have shown a neuroimmune vicious circle in the initiation of pruritus. In psoriasis, accidental denervation of a psoriatic plaque reduces inflammation, suggesting that neurons mediate this inflammation. A recent article(Deng et al., 2024) proposes that the immune system and the nervous system act in concert in the allergic response. It is therefore crucial to develop in vitro skin models that reproduce cutaneous innervation.
To achieve this, a collagen matrix combined with chitosan is used to create sufficiently large pores between the collagen fibres, creating a niche for the cultured cells. The primary cells used come from abdominoplasties, including endothelial cells and fibroblasts, which are initially seeded in the matrix. Sensory neurons from the dorsal root of the spinal cord of mouse embryos were then added to the culture.
This cell culture model was validated using cell differentiation markers such as keratins 10 and 14, showing a three-dimensional organisation similar to that of the human epidermis. This model can be enriched by seeding other cells such as dendritic cells (derived from monocytes) or endothelial cells. By combining this model with a microfluidics platform, it is possible to reproduce vascularisation and study microcirculation.
The skin model innervated in this way responds appropriately: stimulating neurons with capsaicin leads to an increase in TNF production, which is expected in physiological conditions. Similarly, in response to stimulation by cytokines IL-4 and IL-1, inflammatory cytokines such as IL-6 are secreted.
The use of human neurons is also conceivable. In this case, it is necessary to add Schwann cells to obtain dendrites as far as the epidermis. In this model, the sensory neurons are well differentiated, with heterogeneity in the population between peptidergic and non-peptidergic neurons.
The development of this complete, three-dimensional cell culture model opens up a wide range of applications. For example, it has been used to study the immune response of the skin following the bite of arthropod vectors of arboviroses. The virus can be recovered when a mosquito bites artificial skin and injects its virus-carrying saliva. In immunohistochemistry, the areas of infection in the reconstituted skin showed an interferon signature, making it possible to study the overall pathophysiology of arboviral infection in the skin.
In short, it is possible to generate human skin equivalents including immune cells and sensory neurons. This opens the way to numerous applications, such as studying the pathophysiology of arboviral infection in the skin.
Morgan Dos Santos, Labskin Créations, Lyon
The skin was the first organ to be reconstructed in vitro, initially using two-dimensional cultures of keratinocytes, then human reconstructed epidermis, and finally models containing both dermis and epidermis. In recent years, these models have become more complex, with the addition of specialised cells such as endothelial, pigment and nerve cells. Several strategies have been developed to get as close as possible to in vivo conditions.
The top-down strategy is based on the use of a three-dimensional scaffold in which cells are seeded to proliferate and create their own extracellular matrix. The porosity of this medium creates a microenvironment that is highly favourable to cells. At dermal level, this makes it possible to obtain a matrix rich in elastin fibres, which is difficult to obtain with conventional cultures. Equivalent dermis can be enriched with endothelial cells to induce endothelialisation, or even vascularisation, using microfluidic models. Cells are extracted from skin biopsies using the CD31 marker specific to endothelial cells. Lymphatic cells - a subpopulation of endothelial cells - that form lymphatic vessels can also be isolated using the specific marker podoplanin. Capillaries form spontaneously in the papillary dermis, developing a well-defined lumen within these structures, which then establish connections to form a network. The complexity of the model can be increased by incorporating immunocompetent cells, generated from a CD34+ subpopulation derived from cord blood, included during the keratinocyte proliferation phase. Langerhans cells in the epidermis are derived from this population.
The functionality of the included cells was then studied under UVA and UVB exposure. After a dose of UV, the cells become mobile and clump together in the deep dermis.
Skin appendages represent another important dermal component. It has been possible to reconstitute sebaceous glands using sebocytes generated from induced pluripotent stem (iPS) cells. The glands are organised into acini, similar to their structure in vivo, and are functional. Confocal microscopy revealed that the glands were organised to express mucin in the centre and cytokeratin 7 in the periphery, in line with physiological conditions. Under the effect of arachidonic acid, the size and maturation of the glands vary in proportion to its concentration.
The other strategy, bottom-up, uses very small elements to reconstruct a three-dimensional structure. These approaches include the use of dermal and epidermal sheets and 3D bioprinting. The self-assembly strategy enables sheets to form under specific culture conditions, where the cells synthesise their own extracellular matrix. However, the sheets are very thin and need to be joined together. This makes it possible to create a healing model, for example by creating a wound and studying the re-epidermalisation process. 3D bioprinting uses a bioink deposited drop by drop by a machine, according to a pre-established computer model, making it possible to obtain very precise shapes, more complex models and greater reproducibility and speed than traditional methods. The formulation of the bioink is the most complex stage, requiring years to develop a composition comprising fibrinogen,alginate and gelatin, the latter serving temporarily as a scaffold during printing. 3D printing techniques include:
Thanks to these technical advances, it is now possible to create models with three layers (hypodermis, dermis and epidermis), and to vary the cross-linking in order to obtain mechanical properties close to those of physiological tissues. These models open the way to numerous applications, such as grafts for burn victims, as well as fundamental or toxicological research.

Bioderma Congress Reports CARD 2023