4 professionals
Updates on Dermatology: Sun-induced damages
Updates on Dermatology: Sun-induced damages
Get access to exclusive dermatological services to increase your professionnal knowledge: +500 pathology visuals, clinical cases, expert videos
Benefit from valuable features: audio listening, materials to be shared with your patients
Stay informed about the upcoming events and webinars, latest scientific publications and product innovations
Already have an account? login now
Summary of the Bioderma Symposium held during the World Congress of Dermatology in Singapore in July 2023: "Sun-induced damages on the skin: what’s new to protect our patients?" with Prof. Passeron, Prof. Schalka, Prof. Leone and Dr. Fauverghe (NAOS).
Related topics

Stéphane FAUVERGHE
NAOS International Medical Director
Dear All,
I am very pleased to present you the seventh edition of BIODERMA Updates Series dedicated to updates in Dermatology.
For 3 years now, BIODERMA has been regularly organizing international events dedicated to Dermatology, for dermatologists and all healthcare professionals interested in Dermatology, always presented by renowned experts in their field.
In our approach to promote the development of knowledge in Dermatology, we have the pleasure to propose you this new publication, that is the summary of the BIODERMA Symposium held during the World Congress of Dermatology in Singapore in July 2023: Sun-induced damages on the skin: what’s new to protect our patients? with Thierry Passeron from France, Sergio Schalka from Brazil, Giovanni Leone from Italy and myself as speakers.
During this symposium, Thierry Passeron presented: Hyperpigmentation: new benefits from the research for the patient. Sergio Schalka delivered a lecture about the long-term effects of UV on the skin. The lecture of Giovanni Leone was about the emerging role of secondary photoprotection. And, finally, I presented: Ecobiological approach of sun protection: to reinforce the natural mechanisms of the skin.
I wish you all an enjoyable, enriching and interesting reading.

Dept. of Dermatology, University Hospital, Nice, FranceINSERM U1065, C3M, Nice, France
Pigmentary disorders affect up to 60% of subjects, depending on countries and studies. They are among the most frequently reported skin disorders, with an increased prevalence among dark-skinned individuals(1-3). By altering the quality of life they generate a strong therapeutic demand and a suitable diagnosis, based on whether hyperpigmentation is melanic or non-melanic, is necessary to respond to this demand (Figure 1)(4).
Hyperpigmentation disorders are not all linked to an increase in melanin, whether eumelanin or pheomelanin. Skin color is also determined by hemoglobin and carotenoids. Dyschromia can also be due to the abnormal accumulation of other endogenous or exogenous pigments, such as bilirubin or silver. A practical approach is therefore needed to differentiate among pigmentary disorders, whether:(1) a vascular problem, as in the case of hypochromic Bier macules, where the change in color disappears with pressure;(2) xanthoderma, which includes jaundice (increase in bilirubin), and carotenoderma (increase in carotenoids due to high food ingestion, elimination dysfunction or systemic diseases;(3)) exogenous ochronosis, resulting from the chronic use of hydroquinone-containing blanching products;(4) dyskeratosis:(5) chromhidrosis and pseudochromhidrosis (abnormal discoloration of the sweat), caused by topics, clothes, or bacterial proliferation;(6) dirt dermatitis/dermatosis terra firma forme which can be removed by vigorous swabbing with alcohol;(7) heavy metal deposit: check medical history and extra-cutaneous signs; and(8) exogenous pigments, due to trauma, radiotherapy or tattoos.
Melanin hyperpigmentation includes epidermal hypermelaninosis, epidermal hyper melanocytosis, dermal hypermelanocytosis, and dermal hypermelaninosis (pigmentary incontinence). With melanic hyperpigmentation (Figure 1), check whether the lesions are congenital or acquired, look at the pattern of the dyschromic lesions, ask whether topics have been applied, and do a Wood’s lamp examination.
Figure 1. Practical approach to differentiating pigmentary disorders(4)

More than 170 genes are involved in the control of human skin melanin pigmentation (Figure 2). The key factors for skin pigmentation include:(1) number of melanocytes;(2) quantity and quality of produced melanin; (3) dendricity;(4) transport and transfer of melanosomes;(5) localization of the pigments within the skin;(6) elimination rate and/or degradation of melanin; and(7) melanosome pH. Melanin is produced by melanocytes within specific organelles called melanosomes that are then transferred to the surrounding keratinocytes.
Melanosomes travel upwards throughout skin layers, releasing melanin. Depending on their maturation, there are 4 types of melanosomes, with type III mostly present in fair skin, and type IV in dark skin.
Melanin occurs in two primary forms. Eumelanin exists as black and brown, and is photoprotective, whereas pheomelanin provides a red color, is not photoprotective and can induce free radicals. The color of the skin is mainly due to the subtle mix of these two types of melanins. Upon sun exposure, the induced DNA damage within keratinocytes activates the gate-keeper protein p53 which binds to the POMC promotor, thus triggering the release of αMSH which binds to the MC1R receptor on the melanocyte, then activates a pathway leading to an increase in tyrosinase in melanosomes. These melanosomes are transferred to the extremity of the dendrites and then to the surrounding keratinocytes, leading to tanning.
Figure 2. Gene expression in human skin pigmentation
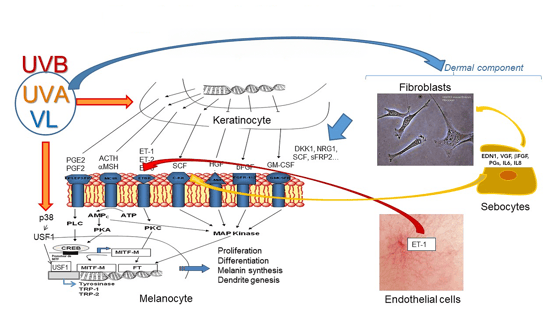
Fibroblasts regulate skin pigmentation and thickness by producing a factor called DKK1(5, 6). They also secrete factors involved in melasma and actinic lentigos, the WNT pathway playing a crucial role in pigmentation regulation by fibroblasts(7, 8). Figure 3 provides an overview of the role of DKK1 in keratino- and melanocytes.
Receptors for nitric oxide, VEGF, endothelin, and prostaglandin receptors are present on the surface of melanocytes(9). A study of 100 benign vascular skin lesions, using high magnification digital dermoscopy, revealed that above dermal vessels there is a restricted but significant hyperpigmentation compared with the surrounding skin(10).
A significant increase in melanin pigmentation associated with skin vascular lesions was observed, confirmed by histology(10). Further experiments demonstrated that endothelin 1, released by microvascular endothelial cells, induces increased melanogenesis signaling characterized by microphthalmia-associated transcription factor phosphorylation, and increased tyrosinase and dopachrome tautomerase levels. Thus, micro-vascularization of the dermis can stimulate pigmentation(10). Such a process appears to be key in several pigmentary disorders, including melasma.
Sebocytes have recently been demonstrated to be one of the actors in melasma pathogenesis, inducing prolonged skin cell stimulation, thus contributing to localized dermal aging and hyperpigmentation(11). UVA-irradiated sebocytes produce and upregulate the factors α-MSH, EDN1, SCF and b-FGF. Sebocyte-derived factors drive modifications of fibroblasts and melanocyte behavior, and sebocytes participate in the skin cell cross-talk regulating pigmentation. Sebocytes locally support the inflammatory and photo-aged environment in melasma.
Figure 3. Molecular signaling of DKK1 in regulating skin pigmentation (5)
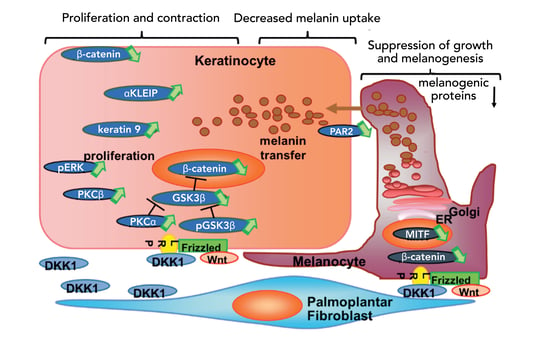
While skin pigmentation regulation is a complex process, it can lead to focusing on new therapeutic targets. The increased knowledge of the melanogenic pathways will help to develop more effective anti-melanogenic compounds. New topical agents could target the dermally-secreted factors, and energy-based devices could help remodel the dermis.

University of Sao Paulo, Brazil
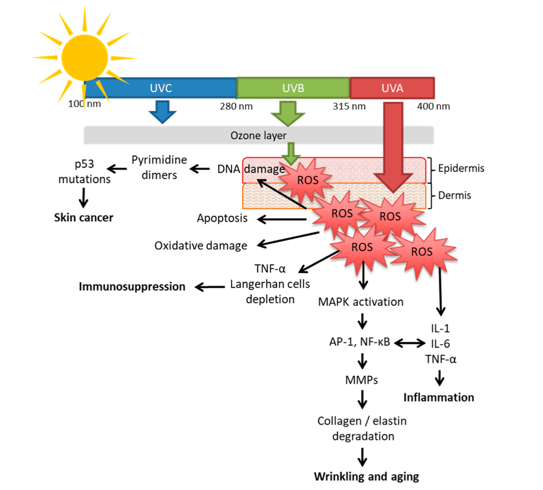
Infrared, visible light and ultraviolet (UVA, UVB) radiations comprise almost all radiations emanating from the sun(1). UVB represents 0.5% of solar radiations, is highly energetic, and was the first portion of UV radiation (UVR) to have its deleterious effects recognized. UVA (9.5% of UVR) is 20 times more available than UVB, less impacted by weather conditions, and penetrates the skin deeper. Visible light (45% of UVR), for which the effects on the skin were discovered more recently, is related to pigmentation and reactive oxygen species (ROS) generation. Infrared (45% of UVR) is responsible for heat sensation and ROS generation (Figure 1).
From the absorption of UV radiations by chromophores to the most relevant clinical features, 3 events are considered central (Figure 2): ROS generation, DNA damage, and immunosuppression(1). Regarding oxidative stress, UVR induces ROS generation in the skin, causes oxidative damage to DNA lipids and proteins, and causes significant damage in the dermis, including destruction of collagen, elastin, and glycosaminoglycans(2). UVB has less influence on ROS production. The peak of ROS generation is in the UVA region (close to 350 nm), and over half of the total ROS generated by sunlight are in the VL region(3).
Figure 1. Solar radiation spectrum(1)

Figure 2. UV and skin: from molecular basics to clinical features(1)
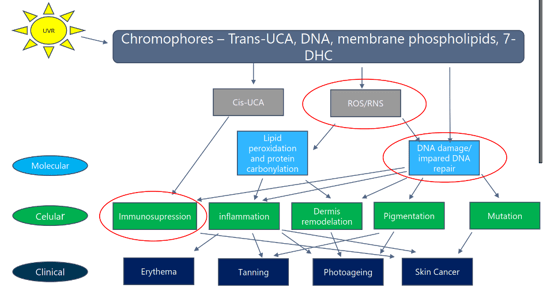
DNA damage can be direct or indirect(4). Direct damage occurs through photoexcitation of DNA bases, with 2 main types of UV direct-induced DNA damage: cyclobutene pyrimidine dimers (90%) and 6-4 photoproducts (10%). Indirect damage is due to DNA oxidation by ROS, leading to formation of the highly mutagenic 8-oxoguanine. The single-stranded break formation occurs mainly through degradation of the DNA sugar units(4). Immunosuppression can occur before erythema with 25-50% of the minimal erythematous doses (MED) in subjects with skin types I/II. Through mechanisms that are not yet fully understood, UVR induces depletion, alterations of morphology, and reduction of Langerhans cells, while UVA-1 induces apoptosis of T lymphocytes(4, 5).
About 70% of the solar radiation received during a typical lifetime is related to daily exposure and not to leisure activities (beach, sport, etc.).
Unlike UVB, UVA radiation is present throughout the day all year long, and is not completely absorbed by clouds or windows. UVAs are sub-classified in short UVA (UVA II, 240 to 320 nm) and long UVA (UVA I, 340 to 400 nm). UVA I represents about 80% of total UV and penetrates deeper into the dermis than UVA II(1, 4). The effects of UVA on the skin include:(1) oxidative stress, UVAI being the most relevant oxidative generator of the solar spectrum;(2) DNA damage, which occurs through 8-oxoguanine formation from DNA oxidation by ROS, direct cyclobutene pyrimidine dimer (CPD) production, and single-stranded break formation. Dark CPD is generated after UV exposure and CPD present in the basal layer instead of upper epidermis as well as impairment of CPD reparation occurs with UVA I + UVB enhancing DNA damage; and(3) immunosuppression as the immunosuppressive action of UVA I is 3 times greater than that of UVB at doses received during normal daily activities(4).
The long-term effects of solar radiation on the skin include keratinocyte carcinoma (KC). There is a relationship between cumulative annual exposure and the incidence of squamous cell carcinoma (SCC). The closer to the equator, the higher the incidence of basal cell carcinoma (BCC) and SCC in men and women. Areas more exposed to the sun (face and neck) have a higher rate of BCC, SCC, and actinic keratosis (AK) lesions(6). However, there is no clear association between sun exposure and KC development in skin of color (SOC)(7). For East Indians, some risk factors include working in outdoor jobs, living at lower latitudes, and greater cumulative sun exposure. Thus, while sunscreen use to prevent KC is recommended for East Asian populations, it is not completely supported by the literature for other SOC(7). High intermittent sun exposure increases the risk of melanoma by 60%, intermittent exposure increases the risk of superficial spreading melanoma, and chronic exposure that of lentigo maligna melanoma(8). A history of sunburn doubles the risk of melanoma, while indoor tanning increases that risk by 20%, with a dose-response effect. High SPF sunscreen use decreases the risk compared to no use, but the risk may increase if sunscreen is used to prolong intentional sun exposure(8).
The sun also plays a role in photoaging. While exposome is the triggering factor in photoaging, ROS generation plays a central role, and sun radiation (particularly UVA) is the most relevant element(9). The wrinkling process is delayed in darker skin types, compared to Caucasians, and a 10-year delay has also been found between Chinese and European populations(10).
Photoprotection is a set of measures aimed at reducing sun exposure and preventing the development of acute and chronic actinic damage through oral photoprotection, regular use of sunscreen, and protective methods such as clothes, shades, etc.(1). The first sunscreens were developed at the beginning of the 20th century to protect fair-skinned individuals from sunburn. It was only in the 1980s that the effect of UV radiation on skin cancer was first reported, and only in the 1990s that the role of UVA in acute and chronic actinic damage was recognized.
By the end of the 20th century, sunscreens possessed SPF between 15 and 30, but had limited protection against UVA radiation(11).
The ideal modern sunscreen should provide a spectrally balanced absorption profile in line with shade and many types of clothing fabric. The goal is to obtain spectral homeostasis through the “neutral density filter effect”(12). Currently, the close-to-ideal sunscreen has the highest UVA protection possible, with a spectral homeostasis coefficient of 0.89. However, while there is a great availability of efficient UVB filters, there is a gap in terms of UVA filters (especially UVA I) which requires a significant increase in its PF value(13). To minimize this excessive exposure to UVA, an active biological approach may be considered to reproduce the skin’s natural processes. It interacts with skin-intrinsic mechanisms, using natural active ingredients such as antioxidants to help the skin reinforce itself.
There is sufficient evidence of the harmful long-term effects of UV on the skin, especially in photoaging and skin cancer. UVA radiation plays a central role in actinic damage through oxidative stress, direct and indirect DNA damage, and immunosuppression.
While current sunscreens are becoming more and more effective in protecting against UVB, they only provide limited protection against UVA. New technologies entailing active ecobiological mechanisms can amplify protection against the harmful effects of UVA radiation.

Israelite Hospital, Rome, Italy
Since 4000 BC, humankind has always found ways to achieve photoprotection(1). Although evidence-based sunscreens were already developed at the beginning of the 20th century, the safety and efficacy of sunscreen ingredients were first assessed in the 1970s(2). Early sunscreens were designed to protect from UVB, and later from UVA. With increasing research over the past decade on the effect of visible light on hyperpigmentation, the need for photoprotection beyond UV has increased(3).
Photoprotection can be:(1) primary, based on the mechanisms of action of sunscreens, i.e., old filters/new filters;(2) secondary, with actives other than filters that increase the efficacy of sunscreens; and(3) based on natural substances acting as filters and possibly replacing/synthetic UV filters.
Recent new evidence supports the need for photoprotection against wavelengths beyond UV (HEV, visible light, and IRA) and indicates that other factors, such as air pollution, may play a role in photodamage and photoaging(4).
Moreover, there is a growing concern regarding sunscreen safety (endocrine disruption properties, skin penetration)(5, 6). But not only, with environmental pollution becoming a center of interest more and more, there are emerging concerns about the impact of sunscreens on the marine environment (biodegradability, bioaccumulation, hormonal changes and endocrine disruption in fish and food chain, impact on flora and fauna)(7, 8).
Currently, modern sunscreen products frequently combine UV filters with one or more biologically active molecules, called “actives”, which provide photoprotection through mechanisms that are not based on the absorption or reflection of UV rays and act differently from UV filters(9). The protective capacity of these actives relies on their capacity to prevent some of the biochemical and molecular consequences in the skin after exposure to UV radiation and once it has been absorbed. A popular example of such a secondary protective strategy is the use of antioxidants in sunscreen product (10, 11).
Although commonly used, the term “biological filter” is misguided, and the term “secondary photo protection” should be used instead, as it is more appropriate. These biological molecules or mixtures, endowed with direct or indirect sunscreen ability, are able to provide additional beneficial effects (e.g. botanical extracts containing antioxidant moieties). However, their efficacy is still poorly documented(12). While providing some additional protection against photo-immunosuppression, carcinogenesis, and polymorphic light eruption, topical DNA repair enzymes provide almost no protection against sunburn(9, 13). Figure 1 provides an overview of targets for biologically active ingredients.
Nowadays it seems that natural photo-protection may become an alternative to synthetic UV filters(14, 15). The photostability, toxicity, and destructive potential of artificial sunscreens on marine ecosystems have generated controversy regarding their safety. Natural selection and evolution have ensured that plants have developed effective protection mechanisms against ROS and UV, and natural components are increasingly common is sunscreens(14). However, which products can be used for photoprotection, which ones are efficacious, and which ones are in the pipeline for the future? Antioxidants are a good example, with vitamins C and E being the most popular. An up-to-date overview of the use of antioxidants in commercial sunscreens for a better understanding of the advantages associated with their use in photoprotective formulations is provided by Jesus et al., 2023(16). Marine organisms have their own efficient mechanisms of photoprotection. Their chemical structure, UVR absorption properties, and pleiotropic role as bioactive molecules are being investigated(17). Natural marine products, such as mycosporine-like amino acids (despite the low SPF observed when added at low concentration as an UV-absorbing compound in lotions), and scytonemin have antioxidant activity, and may represent an alternative eco-friendly approach to protect humans against UV-induced skin damage(18-22). Several polyphenols and flavonoids isolated from microalgae are emerging as antioxidants and photo-protectants, and luteolin has antimelanogenic properties(23, 24).
Figure 1. Targets for biologically active ingredients(9,13)
Herbal substances may work as UV radiation adsorbents and antioxidants, and potentially have few side effects (Figure 2). Among the most studied herbal substances with proven photoprotective activity are green tea extract, carotenoids, and polypodium leucotomos extract (PLE). They have been shown to increase minimal erythema dose and improve signs of photodamage(25). PLE has been helpful in the global treatment of several conditions including polymorphous light eruption, solar urticaria and melasma, and has been used as an adjuvant to the UVB treatment of vitiligo and the photodynamic therapy of actinic keratosis(25). A synergistic antioxidant effect was observed when a grape pomace extract from Vitis vinifera L was added to a sunscreen system containing UV filters(26). The emulsion was safe and more efficient in protecting skin against UVB radiation, taking approximately 21% more time to induce erythema compared to the extract-free sample(26). The potential use of Bellis perennis extract (BPE), also known as the common daisy, was evaluated in cosmeceuticals as a photoprotective factor, using an in vitro model of UVA-induced keratinocyte damage, with results showing photoprotective and immunomodulatory effects of BPE on skin keratinocytes(27). Olive leaf extract is an effective photoprotective, anti-mutagenic and antioxidant active that has shown a synergistic effect in association with UV filters, with an in vitro improvement of the SPF of sunscreen formulations(28).
Ectoine is a compatible water molecule-binding solute (osmo-protectant) produced by several bacterial species in response to osmotic stress and unfavorable environmental conditions. This amino acid derivative can accumulate inside cells at high concentrations without interfering with natural processes and protects the cell against radiation or osmotic stress(29). Ectoine-containing sunscreens have been shown to efficiently prevent DNA lesions induced by both visible and UVA/visible irradiations(30). Ectoine has also been shown to have a protective influence on DNA during electron irradiation(31).
An up-to-date overview of the sunscreen market regarding the use of natural ingredients in sunscreen formulations, with a great potential for the cosmetic industry, was provided by Resvende et al., 2022(32).
Figure 2. Herbal substances offering a potential protection from UV radiation(25)

The effectiveness and safety of natural substances able to provide photoprotection has to be investigated further. As their SPF is rather poor, they should never be used alone but in synergy with chemical filters. So far, no entirely natural UV filter has been officially approved for the EU market. Secondary photoprotection, based on ingredients different from filters, can increase the global photoprotective efficacy of a sunscreen. Finally, the best objective that can be achieved at this time is to combine chemical/physical filters with natural active compounds, thus working with different mechanisms on the post-exposure cascade of events.

NAOS Medical Director, Lyon, France
Since its beginning, BIODERMA has chosen an ecobiological approach to develop products that favor biomimetic ingredients and act on skin-own mechanisms and causes rather than on clinical signs alone.
The UV spectrum is mainly composed of UVA, and particularly of long UVA, responsible for photo-aging and skin cancers. Current sunscreens are more effective against UVB than UVA. While a SPF50+ sun care product stops the direct effect of UVBs in the short term, 11-15% of UVA still reaches the skin with remaining long-term effects via Reactive Oxygen Species (ROS). Due to this imbalance in the skin defense system, it is important to preserve the skin homeostasis.
BIODERMA'S Sun Active Defense approach to skin reinforcement includes the Photoderm® solution, a patented combination of UV filters and biological protection (ectoine plus mannitol), aimed at compensating ROS-induced intracellular DNA damage, and maintaining skin immune system vigilance. Regarding external protection, 90% of the area under the wavelength curve (290 nm - 400 nm) is covered. In in vitro studies, a combination of ectoine plus mannitol was shown to have a protective effect on epidermal Langerhans cell functionality(1). Adding ectoine and mannitol further inhibited the oxidative stress generated by UVAs(2).
In in vivo studies, trans-UCA protection increased by 24% (p<0.05) when ectoin and mannitol were combined with external protection (filters) after UV irradiation, and catalase activity was further preserved by 31%(3). Sun Active Defense provides strong DNA photoprotection against UVAs(4).
For many years, the gold standard for the dermatological treatment of hyperpigmentation has been the Kligman’s trio, consisting of 3 ingredients (hydrocortisone, retinoic acid and hydroquinone)(5). This trio has shown its efficacy overtime but can only be used for 2 months a year maximum twice a year, leaving patients without treatment for 2/3 of the year. Moreover, after treatment with the Kligman’s trio, relapses and hypersensitivity effects, such as irritation or hyperpigmentation, can be observed.

BIODERMA’s ecobiological approach to treating hyperpigmentation intends to extend and improve hyperpigmentation treatment efficacy to last all year long. There are two types of ingredients in the Pigmentbio® product range, those for dark spot efficacy, the LumirevealTM technology inspired by the Kligman’s trio, and a combination of vitamins acting as skin barrier support to optimize skin recovery. Regarding dark spot efficacy, LumirevealTM technology mimics the Kligman’s trio mode of action, with glabridin as an anti-inflammatory, EpidermactivTM to accelerate cell renewal, and andrographolide plus azelaic acid to decrease melanin level in the epidermis.
Skin barrier support is provided by a combination of vitamin C and vitamin E, of which the antioxidant properties help prevent skin ageing and melanin darkening, and niacinamide which reinforces it. Pigmentbio® C-concentrate is a good example of the Pigmentbio® line. In addition to the LumirevealTM technology and 2% fresh vitamin C to reduce dark spots, the C-concentrate contains 2% salicylic acid and 8% glycolic acid, for a “peeling-like” effect, and 2% niacinamide plus 0.5% vitamin E for skin hydration and to prevent visible signs of ageing.
In one study, 34 women, of skin phototype III to IV, with melasma, were treated for 2 months with 4% hydroquinone and Pigmentbio® Daily Care SPF 50+(6). After 2 months, subjects went off hydroquinone and continued Pigmentbio® Daily Care SPF 50+ and applied Pigmentbio® C-concentrate in addition for 3 months as a relay treatment(6). The melasma area and severity index (MASI) score decreased by 10% at the end of the 2-month treatment period and continued to decrease while the subjects were only using Pigmentbio® Daily-care SPF 50+ plus Pigmentbio® C-Concentrate. At Month 5, the MASI score had decreased by 25% compared to baseline(6). In another study involving 41 subjects with melasma, the MASI score was divided by 2 after 3 months of sun care protection and Pigmentbio® C-Concentrate(7). Used as a monotherapy in clinical studies, Pigmentbio® C-Concentrate provided skin hydration in 90% of patients(7), acted as an 8-hour moisturizer(8), and tolerance was 100%(9).
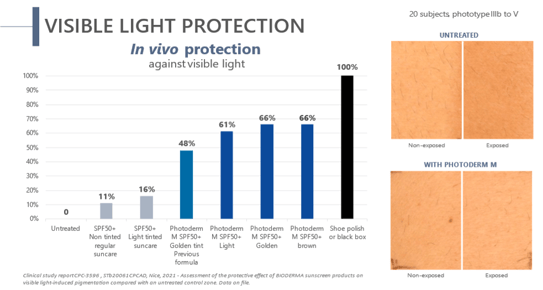
Blue light is directly involved in melasma. High-energy visible light directly activates melanocytes and triggers persistent pigmentation. However, nature is known to deliver yellow pigments, such as lutein and beta-carotene, to the organs most exposed to blue light. Therefore, yellow pigments are bio-inspired skin protectors. With 2 hours of sun exposure being enough to worsen melasma, compliance is key to prevent melasma recurrences. The challenge is to find a galenic product that suits consumer needs to ensure daily use. BIODERMA has developed Photoderm® M SPF50+, a blue light protection sunscreen with anti-recurrence efficacy, high coverage, and a matte finish for dark spots and melasma. The sun active defense in Photoderm® M SPF50+ is based on the combined effects of SPF50+, UVA39, and a clean filtering association. By combining pigments (10.7%) and iron oxide, 61 to 66% of blue light reaching the skin is blocked
(Figure 1)(10). A significant decrease in the MASI index (-31.8%) (p<0.001) was observed after 4.5 months in subjects with melasma using Photoderm® M SPF50+ (11). In addition, the cosmetic qualities of Photoderm® M SPF50+ generated better patient compliance. In one study, 97% of the patients felt better from day to day, thanks to the high coverage of pigmentary spots(12). According to 89% of patients, Photoderm® M SPF50+ leaves a pleasant powdery finish, and 93% said that it held well throughout the day(13, 14).
Photoderm® solution, Photoderm® M SPF50+, and Pigmentbio® C-Concentrate propose an ecobiological approach mixing high efficacy, high tolerance, and high compliance for the well-being of the skin and that of the patient. By combining high technology and sensory appeal, daily application is promoted, thus increasing the chances of success in preventing hyperpigmentation and its recurrence.
Figure 1. In vivo protection against visible light - Photoderm M SPF50+



Updates on Dermatology: Skin disorders



Updates on dermatology: Oncology



Updates on Dermatology: Hyperpigmentation