3 professionals
Bioderma Congress Reports EADV 2025
Bioderma Congress Reports EADV 2025
Get access to exclusive dermatological services to increase your professionnal knowledge: +500 pathology visuals, clinical cases, expert videos
Benefit from valuable features: audio listening, materials to be shared with your patients
Stay informed about the upcoming events and webinars, latest scientific publications and product innovations
Already have an account? login now
Reports written by Dr Anna Zalewska-Janowska (Poland), Dr Nicolas Kluger (Finland), Dr Oriol Yelamos (Spain)

Related topics
Report written by Dr Anna Zalewska-Janowska (Poland)
Chairs : Pr. Branka Marinovic (Zagreb, Croatia), Pr. Marie-Aleth Richard (Marseille, France), Pr. Dr. Martin Röcken (Tübingen, Germany)
Speakers: Dr. Seemal R Desai (Plano, United States), Dr. Marc Scherlinger (Strasbourg, France), MD PhD Masayuki Amagai (Tokyo, Japan)
How to reach unity in dermatology
Speaker: Pr. Dr. Seemal R Desai
The first lecture was presented by the immediate past president of the American Academy of Dermatology (AAD) dr Seemal R. Desai from the University of Texas Southwestern Medical Center in Dallas entitled “Why unity is important in dermatology?” Professor Desai shared with the audience his enormous experience of what his leadership looked like in quite a challenging period. He pointed at dermatology being at the crossroads of medicine, science, and society. Challenges for our specialty are enormous such as rising skin cancer incidence, access to care disparities, burnout among physicians and misinformation on numerous procedures including cosmetics, pigmentary disorders, or skin lightening. The speaker cited Helen Keller saying, “Alone we can do so little, together we can do so much.” Absolutely true! Unity allows us to address all the challenges we encounter - TOGETHER. Professor Semal underlined that unity does not mean uniformity, not at all. He pointed at shared purpose, collaboration across cultures, specialties, and continents. At the same time diversity of skin types and populations, practice settings (academic, private, and public health) together with professional perspectives should be respected. The speaker acquainted the audience with his thoughts about why unity matters in dermatology. He cited patient-centered outcomes because united voice improves patient advocacy and global health impact because skin diseases are among top causes of disability worldwide, education and research because cross-border collaboration accelerates discovery and finally professional strength because united specialty is stronger in the face of political, economic and societal pressures. Professor Desai cited a few more leaders including Martin Luther King Jr and Nelson Mandela. The speaker also focused on the advice to embrace every opportunity and be present, build relationships and develop own reputation by authenticity and ethics at work, commitment, reliability, and outcomes. The talk was very humanistic and brought to the audience a lot of food for thought.
CAR T-cell therapy in autoimmune diseases
Speaker: Dr. Marc Scherlinger
The second lecture was delivered by dr Marc Scherlinger from Rheumatology Department in Strasbourg in France entitled “CAR-T cell therapies in autoimmune diseases.” The speaker pointed out the true need for new treatments in autoimmune diseases giving examples of SLE where remission, regarded as the phase off treatments is achieved in about 25% of patients whereas in myositis in about 20% to 40% of the patients. Dr Scherlinger reminded the audience that B cells are a therapeutic target in autoimmunity. He demonstrated that Rituximab (chimeric anti-CD20 antibody) has some therapeutic limits in selected autoimmune diseases due to incomplete tissular B cell depletion and terminally differentiated B cells sparing effect. The speaker presented his thoughts on how B cell depletion can be improved and pointed at 1. improvement of existing monoclonal antibodies (mAbs) directed against CD-20 (anti CD-20) namely rituximab and obinutuzumab, 2. targeting CD-19 (inebilizumab) and 3. novel immunotherapies such as CAR T cell – anti CD-19. He pointed at the idea of joint targeting methods such as CD19/BCMA, however also stressed that careful identification of the patient who may benefit from BCMA targeting (i.e., plasma cell autoimmunity) should be performed. Pathogenic B cells in autoimmunity are different in different autoimmune diseases such as ANCA-vasc, lupus, Sjögren syndrome etc. There are also safety issues as regards CAR-T cells therapy in systemic autoimmune diseases, namely in acute phase of the disease, the therapy is well tolerated but risk of secondary lymphoma does exist - it is low but still present. There are also questions concerning teratogenicity. Dr Scherlinger described in detail how the technology of CAR could be easier (in detail Lancet Rheumatol 2025 Jun; 7;(6)). In general CAR therapies target antigen-specific B cells. The speaker presented example from literature of different CAR therapies such as an IPSC-derived CD19/BCMA CAR-NK therapy in a patient with systemic sclerosis. He also pointed out easier and cheaper alternatives to CAR therapies such as T-cell engagers (TCE) namely CD3xCD19 T cell engagers. Already bispecific T cell engager therapy for refractory rheumatoid arthritis (blinatumomab) was introduced. So, in conclusion, novel intensive immunotherapies represent an opportunity for autoimmune patients to allow them to function in society.
From tolerance to therapy: Harnessing inducible regulatory T-cells for precision control of autoimmune disease
Speaker: MD, PhD Masayuki Amagai
The final lecture in the session was delivered by Professor Masayuki Amagai from Keio University School of Medicine in Japan entitled “From tolerance to therapy: Harnessing iTregs for precision control of autoimmune disease.” The speaker presented the pathway of his and his group achievements as regards pemphigus vulgaris discoveries. Breaking through article was published in Cell in 1991 when pemphigus vulgaris was described as anti-Dsg3 IgG mediated life-threatening autoimmune disease. Dsg3-specific CD4 effector T cells were discovered to be commander in chief responsible for pemphigus antibody production. Then development of pemphigus vulgaris mouse model using autoantigen knockout mouse followed which was a break-through in pemphigus vulgaris research (Amagai et al J Clin Invest 2000). The speaker presented the way how tolerance to Dsg3 is developed. Of importance, isolation of Dsg3-specific T cells that help B cells produce pathogenic IgG and induce Pemphigus vulgaris phenotype was demonstrated (J Immunol 2008). The speaker acquainted the audience with the vast knowledge on how central and peripheral tolerance is developed. The full process of chimeric status generation in Dsg3 expression in thymus and skin was published by his Lab Group in PNAS in 2021. Of note, Dsg3-specific T cells are eliminated in the periphery, Tregs play an essential role in the deletional peripheral tolerance. Then, the presenter focused on the broad issue – from tolerance to therapy i.e., whether we can convert pathogenic anti-Dsg3 CD4+ Teff cells to anti-Dsg3 Treg cells in pemphigus vulgaris patients aiming at iTreg therapy for pemphigus. It is a new therapeutic approach. Thanks to the work of Mikami et al published in Proc Natl Acad Sci in 2020 on the new method to improve iTreg instability the way for exciting discoveries was paved. Dsg3-specific s-iTreg demonstrated antigen-specific immunosuppression in vitro and maintained higher gene expression of important immunoregulatory molecules than WT S/F-iTreg. A set of careful experiments revealed that Dsg3-specific s-iTreg suppressed B cells in an antigen-specific manner and clinical severity was significantly suppressed by Dsg3-specific S/F-iTreg in pemphigus mouse model. So, S/F-iTreg suppressed autologous CD4+ T cells proliferation. Recent results of these breathtaking results are already accepted for publication in Sci Transl Med (Mukai et al).
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
From posters by:
Ilse Moreno-Arquieta et al. Skin disorders in Common Variable Immunodeficiency (P3272)
Common variable immunodeficiency (CVID) is a primary immunodeficiency disorder characterized by low levels of serum immunoglobulins and impaired antibody responses due to a defect with B lymphocytes, leading to increased susceptibility to infections. It typically presents in childhood or early adulthood with recurrent respiratory and gastrointestinal infections, and some patients may also develop autoimmune diseases. CVID also leads to an increased risk of lymphoma (P0533; P3272).
A Mexican single center study reviewed the cutaneous manifestations in 40 patients (P3272). Skin diseases were present in 35% of the cases. Infections were the most common dermatologic manifestations (7.5%) with various infections like oral candidiasis, herpes, molluscum contagiosum and repeated pyogenic infections, inflammatory skin disorders (atopic eczema, dyshidrosis, seborrheic dermatitis, rosacea) and autoimmune disease (vitiligo, lupus).
Although unspecific as such, it is important to remind that cutaneous manifestations are associated with immunodeficiencies of childhood and adult onset. Of note, TNF alpha inhibitors should be avoided due to the increased risk of infection. The possible association of CVID to inflammatory bowel disease limits the use of IL17-inhibitors in this situation. IL-23 inhibitors could have a place in case of necessity, as illustrated in the case of 38 yo with psoriasis and CVID that benefited from Guselkumab therapy without increased infection (P2074).
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chairs: Prof. Dr. Alexander Katoulis (Greece), Prof. Dr. Ozlem Dicle (Türkiye)
Speaker: Dr. Maryanne M. Senna (United States)
Pseudopelade de Brocq: still debating 140 years later whether it is an autonomous entity or the end stage of cicatricial alopecia?
Speaker: Dr. Maryanne M. Senna (United States)
Brocq’s pseudopelade (BPP) is a chronic, slowly progressive scarring alopecia of unknown exact pathophysiology. It was first described by the French dermatologist Paul Brocq in 1884. The nature of this disease remains controversial: some accept that it is a truly primary disorder, while others consider it secondary to lichen planopilaris or cutaneous lupus, or as the end-stage of severe scarring alopecias. In that case, it would represent only a “pseudopeladic” state without identified etiology. For some, the term pseudopelade should be replaced by the broader term patchy scarring alopecia of unknown origin.
It appears to be genuinely rare in specialized trichology consultations. It mainly affects women between 30 and 50 years of age, although some pediatric cases have been reported.
It is characterized by multiple small alopecic patches, rounded or oval, non-inflammatory, white or flesh-colored, asymptomatic or mildly pruritic, and rarely scaly. The vertex and parietal regions of the scalp are most often affected. The lesions usually present as small bald patches, generally 5–10 mm in diameter, grouped in the same area, producing the appearance of “footprints in the snow.” The lesions may increase in number or merge into larger patches by coalescence. They are atrophic, smooth, and without visible follicles. Dermoscopic examination confirms the absence of hair follicles at the center of the patches and the absence of a pink hyperkeratotic halo, which is characteristic of lichen planopilaris.
The disease course is insidious and slowly progressive over several years, with development and coalescence of plaques. Patients often discover their alopecia incidentally. The process seems to stop spontaneously after a few years. Sometimes a scarring alopecia initially classified as BPP may later reveal itself as another scarring alopecia during a subsequent episode—most often lichen planopilaris, more rarely chronic cutaneous lupus erythematosus.
Differential diagnoses at onset include alopecia areata but also lichen planopilaris and discoid lupus erythematosus. Nevertheless, there are no pathognomonic features of BPP, and it remains a diagnosis of exclusion.
The evolution of BPP is chronic, and follow-up is difficult since the condition remains asymptomatic, non-inflammatory, and slowly progressive. Photographic monitoring appears necessary. Medical management mirrors that of lichen planopilaris: topical corticosteroids, intralesional corticosteroid injections, hydroxychloroquine, methotrexate, etc. The course of BBP is more unpredictable than that of LPP, making it difficult to determine the optimal dose, treatment duration, and risk of recurrence. Autologous hair transplantation and surgery may be considered after several years of stable disease.
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chair: Dr. Nicolas Kluger (Finland)
Speaker: Dr. Christa de Cuyper (Belgium)
Piercing consists of the permanent placement of an object that is externally and partially visible, after breaching the skin barrier with a needle.
Like tattooing, this practice is widespread throughout the world. Motivations are varied: social markers (cultural identification, belonging to a social class or ethnic group), rites of passage, sexual orientation, or simply aesthetic purposes, seduction, reclaiming one’s body, etc.
Piercing can be performed in many locations: earlobes, but also the lips, nostrils, auricular helix, eyebrows, navel, nipples, nasal bridge, and genital organs. In practice, virtually any part of the skin can be pierced.
Various materials can be used to make the jewelry: surgical steels, niobium, titanium. Nickel, a highly allergenic metal, is sometimes found in certain alloys. Since 2001, jewelry containing alloys with more than 0.05% nickel has been banned in Europe. Other non-metallic materials are also available: Teflon, Tygon, PMMA.
A very wide range of models is available: rings, straight bars, curved bars, spirals; closed with screws or clips; with or without ornaments (ball, spike, others...).
Piercings are usually performed in a “professional” parlor. The piercer acquires technical know-how and experience that make them a true piercing professional. However, their anatomical and chemical knowledge, as well as their understanding of hygiene and aseptic rules, is generally self-taught.
Healing from a piercing can take several months.
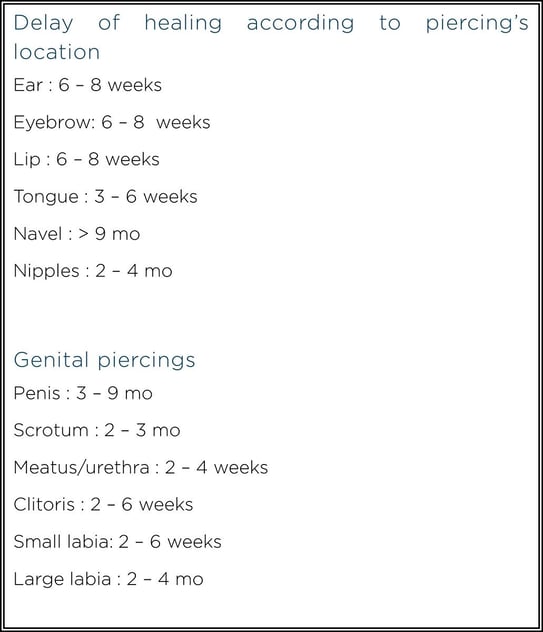
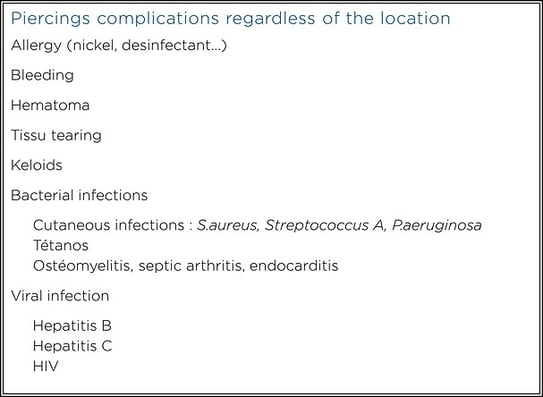
Two types of complications can be distinguished after piercing: those that occur regardless of the site, and those specific to a given anatomical location.
Potential complications depending on the anatomical site (non-exhaustive list)
| Ear | Perichondritis, abscess, traumatic tears, embedding of jewelry |
| Mouth | Problems with chewing and swallowing, perforation, tissue tearing, inhalation or swallowing of jewellery, galvanism, nerve damage, radiological interference, halitosis, gingival recession, speech alteration (lisp), dental damage, Ludwig’s angina |
| Navel | Irritation, rejection |
| Nipples | Disruption of breastfeeding, risk in case of breast implants (?) |
| Nose | Inhalation or swallowing of jewelry, perichondritis, necrosis of the nasal septum, septal hematoma |
| Genital (female) | Misplacement through the clitoris; altered effectiveness of mechanical contraception (condom, diaphragm) |
| Genital (males) | Friction-induced irritation, paraphimosis, penile engorgement, priapism, recurrent condylomas, urethral rupture, urethral stricture, interruption of urinary flow, loss of jewellery during intercourse |
From the posters by:
Gardner-Diamond syndrome (GDS) — also called autoerythrocyte sensitization syndrome or psychogenic purpura — is a psychodermatological entity that remains debated. It is characterized by the appearance of painful, infiltrated, edematous lesions that progress into bruises within 24 hours, without associated biological abnormalities. It primarily affects adolescent girls and young women, usually after psychological stress. There is no systemic involvement. Immunological and coagulation tests are normal. There is no tendency toward capillary fragility (for example, during a cuff test with a blood pressure monitor (P2252)).
Skin biopsy shows dermal edema, a perivascular mononuclear infiltrate, and extravasation of red blood cells without vasculitis or thrombosis.
One of the defining features of GDS is its psycho-emotional dimension, which some consider an important criterion of the syndrome. However, the links between stress, its influence on physiological processes, and immunological changes leading to anti-erythrocyte reactivity have not yet been established.
Intracutaneous injection of the patient’s washed autologous erythrocytes triggers the development of typical lesions the following day.
Management is primarily psychological or psychiatric, particularly in cases of underlying psychological disorders (depression, anxiety, etc.) (P2276). GDS remains an exclusion diagnosis
Figure 1. Gardner-Diamond syndrome. Ilustration generated by ChatGPT

Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chairs: Prof. Nicolas Dupin (France), Prof. Dr. George Sorin Tiplica (Romania)
Speaker: Dr. Sébastien Fouéré (France)
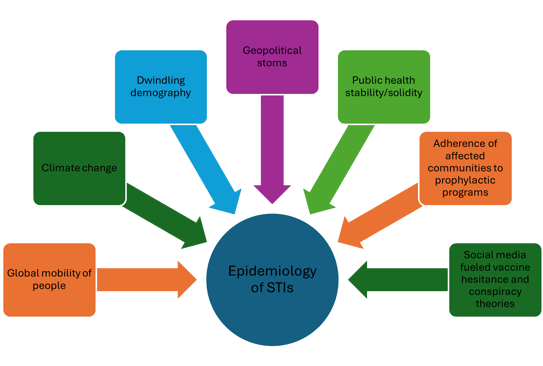
Sebastien Fouéré, head of deparment of the STIs clinic in hospital Saint-Louis in Paris presented the current trends:
Figure 2. Factors that are currently affecting the worldwide trends of STIs
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chairs: Prof. Dr. Alexander Zink (Germany), Dr. Karlijn Clarysse (Belgium)
Speaker: Dr. Alia Ahmed (United Kingdom)
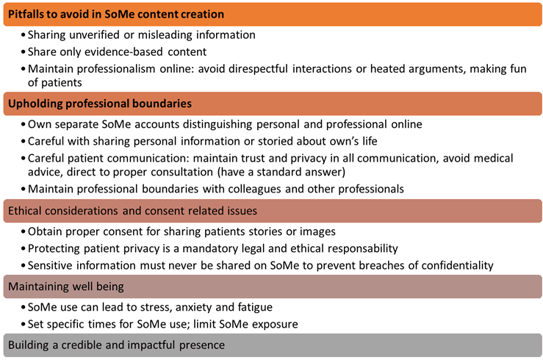
British psychodermatologist Alia Ahmed (@the_psychodermatologist) discussed brillantly the various gray areas associated with use of social media (SoMe) by dermatologists in a daily practice. They include avoiding common pitfalls to protect their professional image and patient relationships, respecting ethical boundaries in order to preserve trust and credibility in dermatology practice and maintaining own’s wellbeing by balancing SoMe engagement (Figure 3).
Figure 3. Dos and Don’ts in Social media (modified from Ahmed)
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chairs: Prof. Michel Gilliet (Switzerland), Prof. Dr. Alexander Zink (Germany)
Speaker: Dr. Josep Malvehy (Spain)
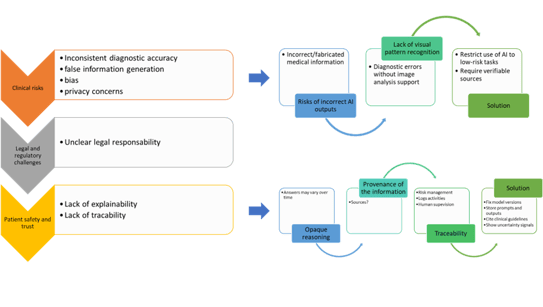
ChatGPT (or others Large language models, LLMs) is not a medical device; it lacks a declared purpose; it is not designed or marketed for clinical use and does not meet the legal criteria under MDR or similar framework. Limitation of current LLM includes: inconsistent patient diagnosis; underperformance compared to physicians; difficulty for following guidelines; poor integration into clinical workflows and unsuitable for autonomous medical decisions (Figure 4). The EU Artificial Intelligence Act (Regulation (EU) 2024/1689) is the world’s first comprehensive AI law. It uses a risk-based approach: AI practices deemed unacceptable (e.g. social scoring, manipulative systems, mass biometric surveillance) are banned; high-risk systems (in our case, healthcare) face strict requirements for safety, transparency, oversight, and data quality. It also sets obligations for general-purpose and “foundation” models, with extra safeguards for those posing systemic risks.
Figure 4. Limitation of current Large language models (LLMs)
Patients should also be aware that a physician may be using AI tools for clinical reasoning and the physician should provide the answer of AI to the patient as well (in the same way a patient would ask for all his CT scans results).
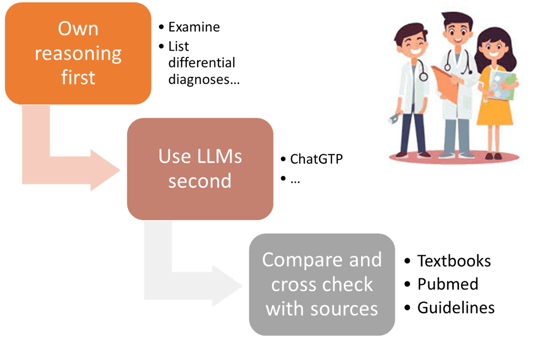
Lastly, the AI may be responsible for diminished skills of physicians and.. medical students or residents. We have evidence that LLM can negatively impact medical doctors’ skills by promoting overreliance, propagating misinformation, diluting critical thinking, and introducing bias. The risk is also important for medical students leading to skills atrophy; motivational decline, pedagogical erosion, ethical risks, social fragmentation and creativity suppression. Proper recommendations should be given to medical students and residents in training to know how to properly use AI LLMs (Figure 5).
Figure 5. Proper recommendations that should be given to medical students and residents in training to know how to properly use AI LLMs
Overall current AI tolls lack diagnostic reliability and have biases affecting skin representation. There are privacy concerns and legal exposure make unregulated AI use in clinical dermatology risky and potentially harmful. Lastly, AI use for educational tasks should be performed under human oversight.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Dr. Paola Pasquali (Spain), Dr. Mayur Davda (India)
Speakers: Dr. Oriol Yelamos (Spain), Dr. Paola Pasquali (Spain), Dr. Mayur Davda (India), MD PhD Konstantinos Liopyris (Greece).
Tips for using images in presentations and publications
Speaker: Dr. Oriol Yélamos (Spain)
This was a very practical session in order to maximise the information we can obtain when we are taking medical pictures. It is important to highlight that if we want to have an image accepted in a journal, it needs to be of quality, therefore we need to maximise the relevant clinical information and minimise the distractors. To summarise, when we take a picture, we should:
Special areas, special uses
Speaker: Dr. Pasquali (Spain)
When we take pictures of the hair sometimes the image gets burnt. Tips to improve it is to reduce exposure and sometimes even not using the flashlight. However, for clinical images of the hair the best would be having a dedicated specialised device since hair photography is very hard.
Another location that is tricky are the fingers. If we want to take a picture of all 5 fingers, it’s best to use a black background to minimize shadows. Also, telling the patient to hold a black cloth can help.
Feet are also difficult. If we are taking pictures of the dorsum, you can use a cloth to use as a background, or make the patient lay down, bend the legs and have the soles lay flat on the bed. Conversely, for the soles either we use a cloth, or we tell the patient to lay on their belly and place the dorsum of the feet on the bed, so we can easily see the soles.
Oral cavity images
Speaker: Dr. Davda (India)
The oral cavity has some particularities which make it difficult to photograph it’s small, closed, has saliva which makes light reflect…
To circumvent these issues, we can use different tools: spacers, mirrors, contrasters…
Also, we need to always use a flashlight to obtain uniform illumination, as well as to be a bit away from the interest area so there’s no distortion of proportions.
Total body photography (TBP) and dermoscopy images
Speaker: Dr. Lyopiris (Greece)
TBP is very useful to monitor patients with numerous nevi. However, if we happen to have the device, why not using it in every patient? Especially if we have standardised systems that take pictures with the same parameters. This will allow for a more consistent comparison over time.
Regarding dermoscopic images, to maximise the image, the best tip is to use alcohol when taking pictures. This will improve the image quality, will standardize focal distance, and will allow us to take polarised and non-polarised images. It is also recommended to take, prior to dermoscopic images, a general clinical image and a clinical close-up.
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chairs: Dr. Cheryl Rosen (Canada)
Speaker: Dr. Cheryl Rosen (Canada)
Hydroxychloroquine is a widely used in dermatology to use various autoimmune and inflammatory diseases like lupus
The main cutaneous side-effect of HCQ is a non-specific drug rash that occurs within 4 weeks of starting the drug. It resolves within weeks of stopping the drug.
Hyperpigmentation is one of the most characteristic cutaneous side effect. It requires a high cumulative dose (> 100 g). Its pathogenesis is possibly secondary to bruising or trauma. The association of hyperpigmentation with concomitant anticoagulation/anti platelet has not been proven with certainty. Hyperpigmentation affects mainly women, taking HCQ for systemic lupus in more than 70% of the cases; The mean time of onset of pigmentation is > 2 years and mean cumulative dose > 200 g. The skin may display a blue-gray, brown, brown-black or gray discoloration. The hyperpigmentation can develop at any part of the body, but it develops mainly affects by order the face (<25%9, the legs (20%) and the hands (<15%). The hard palate is the most often affected in the mouth. The histology is usually non-specific overlapping with others disorders that cause pigment incontinence. The presence of hyperpigmentation is not predictive of retinopathy.
The various cutaneous side effects associated with HCQ is summarized in the table.
|
Common side effects associated with hydroxychloroquine Drug rash Hyperpigmentation Pruritus Stevens Johnson syndrome Acute generalized exanthematous pustulosis Psoriasis |
|
Rare Photosensitivity Urticaria DRESS Erythroderma Erythema multiformie Sweet syndrome Erytheme annulare centrifugum |
|
Hair Hair loss Hyperpigmentation Bleaching |
|
Nails Melanonychia |
|
Mucosa Stomatitis Hyperpigmentation |
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chairs: Prof. Vincent Piguet (Canada), Dr. Diana Rojas Alvarez (Switzerland)
Speaker: Prof. Vincent Piguet (Canada), Dr. Diana Rojas Alvarez (Switzerland)
>> Zika and West Nile
Speaker: Prof. Vincent Piguet (Canada)
West Nile virus (WNV) is an RNA virus from the Flaviviridae family, discovered in 1937 in the West Nile district of Uganda, is transmitted to humans through the bite of a mosquito (Culex), itself infected when feeding on birds. The mosquito can also bite horses and others mammals.
There is an increase in case in Europe especially south and south east of Europe, but it is also the leading cause of mosquito borne disease in the US.
In 80% of the cases, the infection remains asymptomatic. Otherwise, WNV can be associated with isolated fever, neurological symptoms (encephalitis, meningitis, poliomyelitis, retinitis). Other inner organs may be more rarely involved. Cases of coma and deaths have been observed. A maculopapular eruption occurs 5 to 12 days after infection spreading across the torso, face and limbs. The rash is painful or itchy in a one patient out of 3. Other presentations include: vesicles, petechiae in children, psoriasiform papules. The management is symptomatic. There is currently no vaccine available against WMV
Zika virus (ZIKV) is RNA virus from the Flaviviridae family, discovered in 1947 in Uganda and in 1952 in Tanzania, transmitted to humans through the bite of a mosquito (Aedes), itself infected when feeding on non-human primates or from infected humans. Transmission occurs also from blood, perinatal, sexual and laboratory exposure. ZIKV infections peaked in 2016 and cases have again declined after. About 80% of the infected patients are asymptomatic. Most common manifestations include an itchy exanthema of the face, neck, trunk and palms, associated with fever and conjunctival hyperemia. A purpura is noted in 5-10% of the cases. Less common manifestations include bleeding in the mouth or on the gingival facial flushing, edema of the limbs, dysesthesia, palmo-plantar desquamation. Guillain-Barré syndrome is rare but possible. Most of all, ZIKV can be dramatic in a case of pregnancy for the fetus while the symptoms are mild for the mother, and if infection occurs early in pregnancy with brain damage, microcephaly, and congenital Zika syndrome (eye and ear impairments, limbs defects). Management is symptomatic. No vaccine is currently available.
Chikungunya virus (CHKV) is RNA virus from the Togaviridae family, initially located in Africa and Asia but that spread to South America. It is transmitted to humans through the bite of a mosquito (Aedes). Symptoms include fever, myalgias, polyarthralgia/polyarthritis. A morbilliform rash appears 3 to 5 days after the fever and lasts 3 to 4 days. The rash can be asymptomatic in 80% of the patients or mildly pruritic. As opposite to Dengue, there is no hemorrhagic manifestations. A melasma like pigmentation of the nose (“brownie nose” or Chik sign) in patients with darker skin is evocative of the CHKV infection. Variants include freckle-like centrofacial pigmentation, diffuse pigmentation of the face, pinna and extremities, flagellate pigmentation and pigmentation of existing acne lesions have been described in skin of color patients. Vesiculo bullous lesions, Stevens-Johnson like symptoms and punched out deep seated ulcers in the folds, peno-scrotal and perianal areas have also been reported. have been reported with CHKV.
Dengue is the most aggressive among arboviruses. Hemorrhagic manifestations are very common and early during onset of the disease affecting the skin, the eyes and the mouth. The rash presents as an extensive redness with islands of sparing with whitish round dots.
>> Oropouche fever
Speaker: Dr. Diana Rojas Alvarez (Switzerland)
Oropouche virus (OROV) is a RNA virus from the Peribunyaviridae family and endemic in South America. It has been first detected in Vega de Oropouche, Trinidad and Tobago in 1955. As opposite to the other arborviruses like Chikungunya (CHKV) or Zika virus (ZIVK), OROV is originating from Central/South America, and it is found mainly around the Amazon in Brazil. However, it progresses up to north. It has been reported from Brazil and Bolivia to Cuba and Dominican Republic. Imported cases have been observed in north America and Europe. It can be expected that with global warming Mexico and the southern part of the United stated become soon endemic areas. Interestingly before the apparition and spread of CHKV and ZIVK, OROV was the second most common arboviral infection after Dengue. It is transmitted to humans through the bite of a midge, a tiny fly (Culicoides paraensis), itself infected when feeding on sloths, but also possibly birds and non-human primate. The urban cycle with a transmission between humans through Culicoides without the need of an animal reservoir is currently responsible for infections in the cities, in the favelas especially. The incubation is short (3-8 days). Symptoms are initially evocative of dengue with fever, intense headache retro orbital pain. However neurological symptoms like dizziness or photophobia are not in favor for dengue. Skin lesions include an exanthema (20-40%) and petechia and other hemorrhagic manifestations (5%). Conjunctival injection is reported in 20-40%. The rash may disappear and reappear 2 to 4 weeks after the acute febrile phase. Less frequent symptoms include neurological central and peripheral symptoms including Guillain Barré syndrome. Possible materno-fetal transmission and congenital infection with microcephaly is still under investigation.
It is important to note that Culex are also the main insect vector for West Nile virus in Europe, which can raise concerns regarding possible autochtonous transmission of OROV in the future in the Old continent. Furthermore, vector control is difficult as midges are extremely small (1–3 mm), making them hard to detect, trap, or exclude with standard screens or nets. Besides, they breed in moist, organic-rich substrates like decaying vegetation, animal waste, riverbanks, and tree holes. These habitats are abundant and hard to target with larvicides.
Report written by Dr. Nicolas Kluger (dermatologist, Finland)
Chairs: Dr. Andrew Winter (United Kingdom), Dr. PhD Alba Catala (Spain)
Speaker: Dr. Alba Catala (Spain), Prof Dr. Carmen Lisboa Silva (Portugal)
Dermatological manifestations
Speaker: Dr. Alba Catala (Spain)
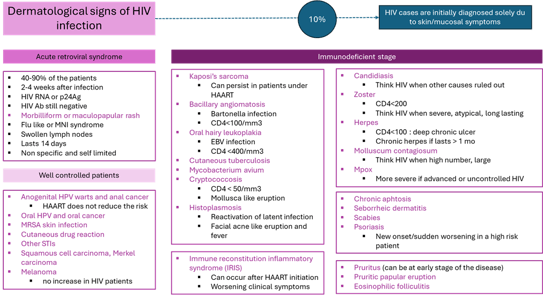
At the time of the past COVID-19 pandemic, Mpox and other emerging arboviruses, it is important to remind that HIV infection still do exist. Cutaneous manifestations of HIV have changed since the emergence of antiretroviral agents and highly active antiretroviral therapy (HAART). Currently drug eruptions, HPV-related diseases, MRSA infections, epithelial tumors and STIs are on the rise in HIV patients. They are recommended to get recombinant zoster vaccine, should be vaccinated against HPV and should undergo regular screening for anal and cervical cancer.
Figure 6. The various cutaneous manifestations of HIV
PrEP for noobs
Speaker: Prof Dr. Carmen Lisboa Silva (Portugal)
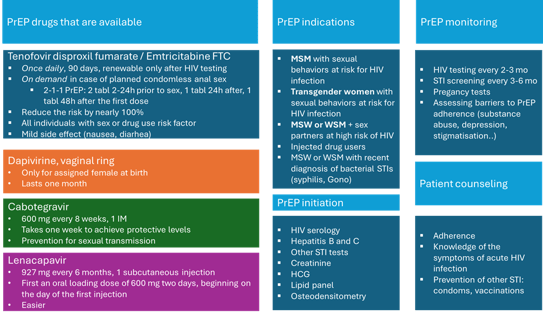
The strategies to prevent HIV include: the use of condoms ; HIV screening; counselling and group interventions; post-exposure prophylaxis (PEP); antiretroviral therapy in HIV+ patients and pre-exposure prophylaxis (PrEP). PrEP is defined by the use of antiviral medications by HIV negative individuals to prevent HIV acquisition. A prophylactic dose of antiretroviral prior to potential exposure to HIV.
The idea PreP :
PrEP includes oral medication (tenofovir), ring in women (Dapivirine) and injectable drugs (intramuscular Cabotegravir or lately subcutaneous Lenacapavir in 2025). PrEP drug should inhibit immediately viral replication upon entry in the body to prevent chronic HIV infection.
Oral and injectable PrEP are inadequate in patients with acute or chronic HIV infection. Such patients must be excluded and PrEP users educated for acute retroviral syndrome’s symptoms.
There is a clear association between efficacy in PrEP and adherence.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Prof. Dr. Eduardo Nagore (Spain), Prof. Danica Tiodorovic (Serbia)
Speakers: Dr. Veronique del Marmol (Belgium), Prof. Danica Tiodorovic (Serbia), Dr. Eduardo Nagore (Spain), Dr. Ana-Maria Forsea (Romania)
Screening and prevention campaigns: are they delivering?
Speaker: Dr. del Marmol
Skin cancer is the most common cancer in Europe, and its incidence and mortality are still growing. Several primary and secondary prevention campaigns have been implemented in different parts of the world, and so far, the only ones to have shown some impacts are the Australian ones. In Australia, incidence and mortality continue to grow but the generations under 35-40 are showing a decrease in incidence, proving that long-term UV exposure prevention campaigns work. Nevertheless, we need a lot of time to see these changes happening, since the campaigns started in the 1980s. Why is that? Because we need to change behaviors (sun avoidance, self-screening…), and this doesn’t happen fast.
What happens in Europe? All the Euro melanoma campaigns have proven that, when active screening (secondary prevention) occurs, we can encounter 20 times more melanomas than expected incidence of melanoma in the general population. The main problem of these campaigns is that, since they are voluntary, the majority of people attending are already more conscious about the importance of skin examination. Therefore, we need to promote education in primary prevention (sun protection and sun avoidance) and secondary prevention (early detection) in all individuals of our society.
Total body and lesion-directed imaging
Speaker: Prof. Tiodorovic (Serbia)
Dr Tiodorovic made an in-depth review of the current evidence of dermoscopy and how it improves skin cancer detection, thus reducing the number of benign lesions excised compared to the naked eye (4 to 20 approximately). She also highlighted the importance of using digital dermoscopy in high-risk individuals to identify thin melanomas in these patients. She also reminded us of the lesions we should never follow with digital dermoscopy (we should excise them instead): nodular lesions, blue lesions, spitzoid lesions and regressive lesions.
She also discussed the newer imaging devices that are the bridge between dermoscopy and pathology such as reflectance confocal microscopy, optical coherence tomography, and LC-OCT among others.
Sentinel lymph node biopsy (SLNB): is it still useful?
Speaker: Dr. Nagore (Spain)
We know we need to stage any cancer and the current American Joint Committee on Cancer (AJCC) guidelines stage melanoma based on the primary tumor, the lymphatic dissemination and the hematologic dissemination. Hence, the most accurate method to test lymphatic dissemination is still sentinel lymph node biopsy (SLNB).
What is clear is that lymph node dissection if positive SLNB is not useful to control melanoma specific survival (MSLT-II trial results). However, SLNB is different: it provides prognostic information – since it determines clinical-radiologic follow up – and identifies candidates for adjuvant treatment.
On the other hand, it can have permanent side effects, since in 5% lymphedema occurs.
The problem is that the prognostication of SLNB is not perfect, but so far it is still the best validated tool we have.
But then, why discuss if SLNB is still needed? Because adjuvant treatment with antiPD1 has been approved already in stages IIB and IIC, so one may wonder why we need to perform SLNB in thick melanomas; the follow up scheme will not change, the indication of adjuvant treatment will not change... But is it ok to treat all IIB and IIC melanomas? We must bear in mind that immunotherapy has also relevant toxicities, so how do we better select which patients to treat? We need to determine the NNT (number needed to treat à who benefits from the treatment) and the NNH (number needed to harm). Also, these treatments are expensive. So, there are still a lot of questions that need to be answered on how to select the best patients to be treated. Maybe integrating clinical and pathological data (nomograms), gene expression profiling and additional test yet to be developed, is going to be the way to go.
Where SLNB is clearly indicated is in melanomas IB and IIA, which encompass many cases and therefore a lot of absolute deaths, although the percentage of mortality is lower compared to thicker melanomas. A new study by Stassen et al. (Eur J Cancer 2025) investigated the impact of SLNB in determining whether to use immunotherapy. They found a number needed to treat (NNT) of 69 for pT1b melanomas and 25 for pT2a to be candidate for adjuvant therapy (when in SLNB tumor burden was >1mm). So, there’s still room for SLNB in a select subset of patients until we have better, more efficient and less invasive methods to prognosticate better our patients.
Identifying high risk groups
Speaker: Dr. Forsea (Romania)
Since melanoma incidence and mortality are expected to go up soon, and we cannot screen the entire population, we need to identify targeted interventions.
The strongest predictor risk factor is the large number of nevi, especially if the nevi are atypical (atypical mole syndrome). Also, lighter phototypes have more risk. Also, eye colour is a good indicator, regardless of the phototype. Hair colour is relevant in red hair individuals.
The personal history of melanoma also increases the risk, especially if more individuals in the family have a melanoma. Around 50% of individuals with familial melanomas have a known genetic mutation. There are also some genetic mutations we don’t know yet.
Also, we need to consider that the individuals at high risk of developing a melanoma are not the same as the individuals with high risk of developing an aggressive melanoma. Patients at risk of having an aggressive melanoma include males over 50, living alone, low socio-economic status, fast growing melanomas (EFG-melanomas à Elevated, Firm, Growing rapidly).
We are getting risk calculators that integrate these data to calculate the risk of melanoma in each patient.
Finally, we have numerous apps that can help us and help patients in assessing their skin. The problem with apps is that most of the time, we don’t know by whom they have been developed, so dermatologists need to lead studies to assess the validity of these AI apps.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Dr. Antoine David Petit (France), Prof. Fatimata Ly (Senegal)
Speakers: Dr. Antoine David Petit (France), Prof. Fatimata Ly (Senegal), Prof. Jelena Stojkovic-Filipovic (Serbia), Dr. Lilia Bekel (France).
Melasma
Speaker: Dr. Petit (France)
Dr Petit reviewed the clinical presentation and treatment options in melasma. He highlighted the importance of sun protection even in darker phototypes since not only UVB is important, also UVA and blue light can induce hyperpigmentation in melasma. It’s now well known that these long UVA and blue light have a stronger effect on melasma on darker skin since the opsin 3 pathway is more active in such phototypes.
Regarding treatment, still, hydroquinone is the key treatment in melasma, which works better when used in the classic triad of a mild steroid and retinoid. However, it’s important to stress that these treatments should be used maximum 3 to 6 months (typically fall/winter season) to avoid side effects (ochronotic, estriae…).
It is important to document correctly the cases to be able to assess the evolution of the treatment, since patients may not be satisfied since the treatment tends to be slow.
As second line treatments, oral tranexamic acid (TxA) 250 or 500 mg bid is a very effective treatment, when having ruled out thrombotic risk. However, there’s an unanswered question which is what we do with TxA when the patient is under control, since if we withdraw TxA completely melasma will reappear. Normally, what people would do is use TxA for 1-2 months and then stop it, although there some studies showing safety after 1-2 years.
Post-inflammatory hyperpigmentation (PIH)
Speaker: Dr. Ly (Senegal)
PIH occurs due to activation of melanocytes by inflammatory mediators, such as trauma, and is activated by UVR. PIH happens in all phototypes but it’s more intense in phototypes III to VI. PIH induces a strong psychosocial distress, especially in countries with the majority of people having dark skin. In fact, PIH is very common and is the 11th most common dermatosis worldwide.
Dr Ly performed a study on the causes of PIH and found in their African cohort that the most common cause of PIH is post-acne. Interestingly, they found no association between the severity of acne and PIH. However, there was an association of PIH and the type of acne (inflammatory) and the manipulation of lesions.
Other factors also can induce PIH, and include skin inflammatory diseases (pigmented rosacea, atopic dermatitis, psoriasis, systemic sclerosis, lichen planus and lupus erythematosus), or exogenous factors besides trauma (phytophotodermatitis, oral medications such as bleomycin or minocycline…).
There are different options for treatment (hydroquinone, azelaic acid…), but the crucial point is prevention with manipulation avoidance and use of specific sunscreens, which can also be physical sun protection and sun avoidance. In this sense, it is important to highlight the importance to tailor photoprotection according to the phototype lighter phototype will benefit from stronger UVB protection (SPF50+), whereas darker phototypes would benefit more from UVA and blue light photoprotection, as well as milder UVB photoprotection (SPF30).
Hyperpigmentation linked to systemic diseases
Speaker: Dr. Stojkovic-Filipovic (Serbia)
Hyperpigmentation can occur in numerous systemic diseases. For example, almost 20% of endocrinologic disorders can present with hyperpigmentation.
We know this is due several causes: hormonal imbalance, metabolic dysfunction, autoimmune processes, deposition of abnormal substances, drug induced…
In this comprehensive and academic session, different causes were reviewed in a systematic manner.
Hormonal imbalance hyperpigmentation can be due to the activation of tyrosinase from different stimuli. In Addison disease and Cushing diseases, this is initiated by ACTH which later activates MITF, later activating tyrosinase and the melanocytes.
Diabetes can also induce hyperpigmentation via different mechanisms but mostly due to insulin resistance and hyperinsulinemia. This leads to different clinical presentations: diabetes dermopathy (typically hyperpigmented macules asymmetrically located on the shins), acanthosis nigricans (typically on the neck) can appear before glucose alterations, diabetic prurigo (often linked to dry skin)
Regarding acanthosis nigricans it is important to highlight that it can be a paraneoplastic condition, which we need to suspect in individuals who do not have diabetes or metabolic syndrome.
Other hormonal diseases that lead to hyperpigmentation include thyroid disorders, especially hypothyroidism. This happens due to an increase of MSH secondary to low thyroid hormones, which lead to generalised hyperpigmentation.
Metabolic disorders can also induce hyperpigmentation but via different pathways:
Nutritional deficiencies can also lead to hyperpigmentation, such as B12 deficiency, or niacin deficiency (pellagra).
Hyperpigmentation in genetic syndromes
Speaker: Dr. Bekel (France)
Several genetic syndromes are associated with hyperpigmented lesions, café-au-lait macules (CALM) and lentigines being the most common ones.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Prof. Maura Picardo (Italy), Prof. Khaled Ezzedine (France)
Speakers: Prof. Maura Picardo (Italy), Dr. Madhulika Mhatre (India), Dr. Liesbeth Delbaere (Belgium), Prof. Khaled Ezzedine (France).
Genetic syndromes
Speaker: Dr. Ezzedine (France)
Hypopigmentation in genetic disorders is due to a reduction in skin pigmentation, and the alteration in pigmentation can occur in the different steps of melanogenesis. It’s not really that there’s a decrease in the number of melanocytes (what happens in vitiligo).
The first group are the disorders of melanogenesis, and include oculocutaneous albinism and its syndromic forms, Chediak-Higashi, Hermanski-Pudlak and Griscelli syndrome.
The most well-known one is oculocutaneous albinism (OCA). It can be relatively easy to diagnose in darker phototypes but can sometimes be difficult to diagnose in skin phototypes I or II, and a key feature to diagnose them is the presence of nystagmus.
The second group is pigmentary mosaicism, in the form of hypomelanosis of Ito. It presents with hypopigmented whorled lines following Blaschko lines present at birth, and what’s relevant is its associating with seizures and developmental delays.
Another well-known genetic disease presenting with hypopigmented lesions its tuberous sclerosis, which presents ash-leaf macules (at least 3), which are sharply demarcated macules. This is completely the opposite to the hypopigmented macules found in NF1, which tend to be less nicely demarcated.
Waardenburg syndrome presents also with hypopigmented patches, poliosis, hypertelorism, congenital deafness, and iris heterochromia.
Tietz syndrome is a very rare syndrome characterized by MITF mutations and presents with generalized hypopigmentation and silvery hair.
Piebaldism sometimes can be confused with vitiligo, but piebaldism is present at birth, is stable, doesn’t spread over time and typically associates with poliosis. Due to KIT mutations.
Nevus depigmentosus à localised hypopigmented patch sometimes with segmental pattern. Stable over time, present at birth but does not show until first exposures to the sun. Does not progress like vitiligo.
So, if you have a vitiligo clinic you need to know these diseases, since they are in the differential diagnosis and most likely you will end seeing some patients with these diseases. Hence, you need to perform a good anamnesis and consider if lesions are localised vs. generalised, present at birth or not, if there are neurological alterations…
Hypomelanosis
Speaker: Dr. Picardo (Italy)
There are numerous causes of hypomelanosis, primary or secondary, but the most common ones we face are secondary and are the ones that were discussed in this session.
Secondary hypomelanosis
So, what do we need to do with secondary hypopigmentation? The most relevant is to treat the underlying cause of hypopigmentation. Additionally, it can be important to use adequate moisturisers. We need to do this since melanogenesis is a high energy consuming process, so if there is a dermatosis melanogenesis stops (like a computer hibernating) and when the skin inflammation is gone, then the computer turns on again and pigment reappears.
Vitiligo and vitiligo-like syndromes
Speaker: Dr. Mhatre (India)
Vitiligo’s prevalence ranges from 0.25% to 4% and the incidence is rising.
The issue is that it has a very strong psychological impact, especially among darker phototypes.
There are numerous genes related to vitiligo.
CD8+ cells attack melanocytes, which leads to oxidative stress. This attack happens due to genetics and environmental factors. We also know that when vitiligo has become stable, it can reappear. This is due to tissue-resident memory (TRM) cells. Hence, targeting TRM cells via the IL15 pathway may be the way to go to prevent relapse.
Five clinical phenotypes of vitiligo have been identified through cluster analysis, and determine its management:
Treatment of vitiligo should first focus on stabilising vitiligo, mostly systemic treatments (steroids mostly) and NB-UVB.
The revolution in vitiligo are JAK inhibitors, both topical and oral. There are multiple trials ongoing using JAK inhibitors, and so far, the only approved is topical ruxolitinib 1.5%, which in the trials shows that improves repigmentation even in acral areas.
There are new drugs in the pipeline for vitiligo:
Surgery (grafts mostly) is used in lesions that have been stable (6 months) and do not respond.
Platelet-rich plasma (PRP) seems promising when used in combination with excimer laser.
And when patients are under control, we need to maintain the response, and it’s suggested to do it with NB-UVB once weekly, topical calcineurin inhibitors twice weekly, patient education and psychosocial support, and fast treatment in case or early relapse.
Drug-induced hypopigmentation
Speaker: Dr. Delbaere (Belgium)
The new anticancer drugs commonly induce hypopigmented lesions.
Immunotherapy (antiPD1, antiCTLA4 drugs) can present with vitiligoid lesions which are associated with better therapeutic response. These lesions do not respond well to treatment and since one of the treatments is NB-UVB, it is not generally recommended in melanoma patients.
Immunomodulating therapies also can induce hypopigmentation. There are some cases associated with dupilumab and secukinumab, but it has not been studied in depth.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Prof. Harvey Lui (Canada), Prof. Mariateresa Rossi (Italy)
Speakers: Dr. Harvey Lui (Canada), Prof. Dr. Angelika Hofer (Austria), Prof. Hiva Fassihi (United Kingdom), Prof. Mariateresa Rossi (Italy).
Photoprotection in 2025
Speaker: Dr. Lui (Canada)
Photoprotection concepts have switched from something fairly simple to something more complex. In the past, we only used photoprotection to prevent sunburn and skin cancer but now we also use photoprotection to prevent photoageing, pigmentary disorders such as melasma, post-inflammatory hyperpigmentation…
There’s strong evidence that sunscreen prevent sunburns, skin cancer and photoageing, but which is the evidence regarding preventing pigmentary disorders? There is very limited evidence with RCT that sunscreens work for these dermatoses. It is intuitive that they may work, but no formal studies have been performed.
What about other dermatoses? In 2025 WHO has declared sunscreens as essential part of medicines especially in people with albinism. This is a very important milestone. It is not important from a scientific standpoint but from a political standpoint.
What about the general population perceptions of sun protection? In the UK 44% of people thought photoprotection was not for them!
Also, what’s interesting is that black people don’t like going to the sun? Why? Because dark skin gets warm and it’s uncomfortable (think what happens when we wear black clothes!).
What’s in a sunscreen bottle? 20% of a sunscreen is filters.
Now we know that not only UV is relevant, since we know visible light also induces hyperpigmentation, and we don’t have enough options of sunscreens protecting from visible light. Also, we don’t have a harmonised “SPF” system for visible light.
Why do people fear photoprotection: because of fear of chemicals, because of endocrine disruption, because of fear it might impact vitamin D deficiency, but also due to its cost.
Solar urticaria
Speaker: Dr. Hofer (Austria)
Solar urticaria patients have an increase of STAT3 in the mast cells, so this opens the door for new treatments.
Recent studies have shown that the triggers are UVA alone (31%), UVA+VL (29%), UVA + UVB (15%), UVA + UVB + VL (12%).
There are no formal guidelines on treatment of solar urticaria. Of course, the first step is to avoid sun exposure, use clothing and sunscreens. Most of the time this isn’t enough, so patients need to use antiH1 non-sedating antihistamines +- leukotriene stabilisers.
The 3rd step is using phototherapy to induce tolerance. The problem is the effect of short duration, so you need maintenance treatment. So, what is suggested is to induce fast hardening with phototherapy for 3-5 days with intense schemes, and then maintenance therapy twice weekly with natural sun exposure or phototherapy sessions.
And the 4th step – if it doesn’t work – is omalizumab. It is off-label but shows 79% response with 50% of patients being symptom-free. It is not a lot, but compared to other treatments, omalizumab is the best option.
In refractory patients to omalizumab, cyclosporine can be used alone or in combination with omalizumab, as well as intravenous immunoglobulins, or plasmapheresis.
Which other drugs do we have to potentially be used in solar urticaria? Dupilumab has been studied in chronic urticaria and some forms of inducible urticaria.
A new drug is being studied in chronic spontaneous urticaria: remibrutinib, a bruton tyrosine kinase inhibitor. This can be a potential good drug for solar urticaria, but we don’t have data yet.
Newer drugs are under investigation (kit inhibitors such as barzolizumab or briquilimab), but what may revolutionise this disease are JAK inhibitors.
Therefore, there’s a need to study these new drugs specifically for solar urticaria, since most of them are studied in chronic urticaria or other forms of inducible urticarial, but not specifically in solar urticarial.
Phototherapy in challenging areas
Speaker: Dr. Rossi (Italy)
Phototherapy is still the first-line treatment for some dermatoses such as cutaneous T-cell lymphoma (CTCL). But it is ineffective in shaded areas such as the axillae, genitalia…if we use full body phototherapy. Hence, using targeted phototherapy can be useful in certain scenarios. Different light devices can be used such as 308 nm excimer laser or 308 nm monochromatic excimer light (MEL).
Which are the most important indications for excimer light? Psoriasis, vitiligo, alopecia areata, mycosis fungoides (MF), atopic dermatitis (AD), granuloma annulare, prurigo nodularis and morphea.
In some of these diseases, such as palmoplantar psoriasis, localised phototherapy can help improve treatment outcomes. Interestingly, excimer laser doesn’t work well for nail psoriasis compared to PDL laser.
In vitiligo, excimer laser can be used in sensitive areas such as the eyelids.
New lamps are being developed using LED lights, which can be smaller than other lights, can last longer and can be adapted to certain body shapes. What about clinical results of LED phototherapy? In vitiligo the results are equivalent when using 308 nm LED vs 308 nm MEL.
What about combination of phototherapy with newer drugs? Vitiligo patients treated with upa vs upa + 308 nm excimer laser showed that the combination was better, without increasing the risk of skin cancer.
Excimer laser can also be used in MF with good results.
Visible light LED doesn’t work in AD, but it may work for psoriasis, yet further evidence is needed.
Chronic actinic dermatitis (CAD)
Speaker: Dr. Fassihi (United Kingdom)
CAD is an eruption characterised by eczematous lesions appearing in older men (>50 years old), typically on the neck and face and sparing areas not photoexposed (behind the ears, neck skin folds, around the eyes, finger webs…).
Histology is going to be eczematous (spongiosis), but it is mandatory to perform a phototest to diagnose it, and frequently patch and photopatch tests are recommended, since allergies are frequent.
The gold standard diagnostic test is the monochromator testing, a type of phototest performed in specialised units. It is important to avoid steroid use for 1 week prior to the phototest.
And what’s new in CAD? CAD is changing since the mean age at diagnosis is dropping, moving from 50-60 yo to 30-40 yo. Also, skin types prevalence is evolving CAD is now affecting more cases among darker skin types. Also, gender is changing, since this new variant can also affect women. So, we have two types of patients with CAD: the classic older men with lighter skin, and the more recently another group of younger (<30), female patients, with an atopic comorbidity.
It’s important to manage well these patients, since they have very relevant rates of depression with even high rates of suicide due to the intense pruritus. Management involves sun avoidance, and thorough sunscreen use. However, since they protect so much from the sun, most of them are vitamin D deficient and they need to be supplemented.
PUVA is not really helpful for the classic CAD in older men, but it seems to work better in the younger forms.
Dupilumab could be a potential treatment in CAD, although we must be careful with the head and neck reactions in these sensitive patients.
JAK inhibitors can also be helpful in these very difficult conditions.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Prof. Emma Guttman-Yassky (United States), Prof. Matthias Schmuth (Austria)
Speakers: Prof. Emma Guttman-Yassky (United States), Prof. Matthias Schmuth (Austria), Prof. Dr. Eric Simpson (United States).
New and upcoming treatments
Speaker: Prof. Guttman-Yassky (United States)
The latest drugs developed have helped a lot better understand the pathophysiology of AD.
Dupilumab opened the translation revolution in AD.
IL13 seems the central molecule in AD. We got this answer thanks to the lebrikizumab studies.
There are some new molecules under development such as IL31 inhibitors, OX40/OX40L inhibitors, among others.
Nemolizumab targets IL31, which has a role in itch and barrier defects in AD. Therefore, nemolizumab works better in patients with more pruritus.
A new exciting target is IL22/IL22R, which is involved in barrier issues and hyperplasia. It can be targeted with Temtokibart. In a phase 2 analysis it seems the best dose is 300mg, with good safety profile (upper respiratory infections).
A new pathway that is interesting is the OX40/OX40 ligand. They are expressed in activated T cells. Rocatinlimab antagonizes OX40 and works well. Although it takes time to be efficient, it persists longer, and in 90% of cases the responses persist, thus suggesting potential disease modification.
Amlitelimab is anti-OX40L which seems promising but only some press release data is available. Regarding adverse effects, it seems the relevant one is site injections, also rhinopharyngitis.
What about oral medications? JAK inhibitors work faster but have some more side effects such as serious infections and potential thrombotic issues, so do not give them after age 65, smokers, women using contraceptives…
STAT6 inhibitors are studied and seem a promising oral molecule.
Role of environment and allergens in AD flares
Speaker: Prof. Schmuth (Austria)
There are 2 types of patients: patients who have flares and patients who are more stable. But what triggers flares? The typical cytokines that are increased in flares are IL14 and IL13. Thanks to basic research studies, we now know that the main cells in flares are basophils and not mast cells, mediated via leukotrienes, and interact with terminal nerves.
Heat also can induce flares via activation of TRPV3 and nerve activation.
Low humidity disturbs permeability barrier function. So, low humidity impairs the skin barrier.
Other external factors that can trigger flares are organic compounds (VOC), air pollutants.
Hence, controlling these triggers can also save patients from using both topical and systemic drugs.
Super-responders and disease modification in AD
Speaker: Prof. Dr. Simpson (United States)
First, what are super-responders? Patients who respond very well. And why are they important? Because it would be ideal to identify them prior to initiate a given drug. Also, if we know if a patient will respond to a drug or to another this would be great to help tailor the treatment plan. The problem is that we don’t have standards on who is a super-responder or not.
Another thing that could potentially be interesting would be how to modify the disease. The issue, compared to psoriasis, is that in AD some patients present spontaneous remission and others don’t, but severity is very variable among patients. Is there something we can do? Since we cannot modify filaggrin gene status, we can probably change disease severity. Why? To control comorbidities. Severe AD patients tend to have severe asthma or rhinitis. Hence, if we control AD severity, then patients may improve their comorbidities.
Which are the benefits of early interventions in AD then? If you treat early, you target resident memory T cell. This is true for psoriasis, but we still need to know how this can affect AD.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Prof. Aleksandra Danczak-Pazdrowska (Poland), Prof. Franco Rongioletti (Italy)
Speakers: Prof. Bartosz Miziolek (Poland), Dr. Marija Geroldinger-Simic (Austria), Prof. Aleksandra Danczak-Pazdrowska (Poland).
Understanding sclerosing diseases
Speaker: Prof. Miziolek (Poland)
Sclerosing diseases share the fact that the skin hardens, but the pathogenesis can be different. Some are only cutaneous (localised scleroderma/morphea), others are systemic (systemic sclerosis), and others can be due to an underlying malignancy (scleromyxedema, scleroderma…)
There has been some news regarding the treatment of these diseases. For localised scleroderma with a limited extent the first line treatment is topical corticosteroids and/or topical calcineurin inhibitors. If no response is obtained, a novel approach that can be used is calcipotriol or imiquimod. Another novel treatment is autologous fat injection. This has been proposed to treat linear morphea/Parry Romberg form to refill the atrophic areas but also works well due to antifibrotic effects.
Scleromyxedema causes skin thickening on hands, face… and often is associated with an underlying monoclonal gammopathy. Internal organ involvement can also happen and can be severe. The first treatment line is intravenous immunoglobulins (IVIG). If it’s not sufficient we can consider systemic steroids, thalidomide or lenalidomide. If still refractory, bortezomib + dexamethasone, melphalan or even bone marrow transplant.
Scleroderma presents with skin thickening typically on the back. There’s no increase of fibroblasts. It can be associated with respiratory infection prior to the skin thickening (type I scleroderma), but also lymphoproliferative disease (type II) or diabetes mellitus (type III). First line treatments include UVA1 or PUVA, secondary treatment MTX +- steroids, but always associate it with physical therapy.
Systemic sclerosis (SS) is a systemic disease which can have severe consequences especially if there’s lung involvement. There’s some disparity among the treatment recommendations between the dermatologists’ guidelines (EADV) and the rheumatologist guidelines (EULAR). In the current EULAR guidelines, in case of skin involvement MTX should be considered first line as well as rituximab, later mycophenolate if it doesn’t improve. Since 2017 there’s been more interest in using rituximab in SS, since B cells seem to play a key role in SS. Although EULAR guidelines do not include phototherapy, the EADV guidelines do recommend it.
For systemic involvement there are numerous new treatments such as nintedanib, which works very nicely in case of lung involvement, but doesn’t work well for the skin. However, the most promising treatment in SS is CAR-T therapy.
Finally, for the treatment of digital ulcers there are recommendations regarding the use of PDE5 inhibitors (sildenafil, tadalafil), iloprost and atorvastatin, but bosentan doesn’t work to treat ulcers. However, bosentan does work well to prevent digital ulcers (PDE5 inhibitors and iloprost also work well to prevent digital ulcers).
Management of systemic sclerosis
Speaker: Dr. Geroldinger-Simic (Austria)
The diagnosis of SS can be challenging since at the beginning the clinical findings are subtle: Raynaud phenomenon, puffy fingers, skin thickening, calcinosis, digital ulcers… We now have some more things we can do to diagnose SS: blood test checking for autoantibodies, nail fold capillaroscopy…
It is important to diagnose SS early due to potential lethal systemic manifestation, being the lung ones the most severe.
So the role of the dermatologist is crucial in SS to diagnose these patients, as well as to provide risk assessment: cases with a more diffuse form tend to have more severe outcomes (progressive systemic sclerosis, rapid involvement, lung fibrosis) as opposed to the limited forms (arms, legs, head) which have late pulmonary arterial hypertension and a slower presentation.
Also, dermatologists can and should treat digital ulcers in these patients (treatment discussed earlier). For Raynaud’s Phenomenon, first line should be calcium channel blockers, later PDE5 inhibitors, iloprost, topical nitrates and, also, it’s important to avoid beta-blockers and vasoconstrictive drugs, as well as to counsel smoking cessation and warming strategies.
Localised scleroderma/morphea
Speaker: Prof. Danczak-Pazdrowska (Poland)
Morphea is a form of sclerosis that only affects the skin, without systemic involvement.
There are potential environmental factors that may trigger morphea: trauma (for example around the breasts), radiotherapy, drugs, infections (Borrelia).
Clinical presentation should be enough to diagnose morphea, since the presentation is typical, with different phases over time:
If unsure about the diagnosis, we can perform a biopsy, but some authors suggest using MRI instead because you can not only diagnose it but also assess the extent and the treatment response.
Linear morphea is a bit more distinct since it can have atrophy and neurological involvement if occurring on the head.
Other interesting findings in morphea are typically the lack of involvement of the fingers (no Raynaud, no alterations in capillaroscopy, no digital ulcers) and the lack of autoantibodies (or maximum you can find positive ANA at low-titers). Therefore, if clinical presentation is typical of morphea and no finger involvement happen, there’s no need to perform additional capillaroscopy or blood test looking for autoantibodies present in SS.
There are some diseases associated with morphea: vitiligo, genital lichen sclerosus (20%, frequent!). This means we must explore the genitalia of patients with morphea to diagnose lichen sclerosis, and if present monitor the genitalia to diagnose potential squamous cell carcinomas occurring on top of lichen sclerosus.
Regarding one of the triggers, Borrelia, we know we can find it to the same extent to other populations without morphea. Therefore, testing for Borrelia should be only done if clinically indicated, not routinely in all morphea patients.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Prof. Michel Gilliet (Switzerland), Dr. Robert Kirsner (United States)
Speakers: Dr. Kenneth Tomecki (United States), Prof. Thomas Bieber (Germany).
New and emerging infectious diseases
Speaker: Dr. Tomecki (United States)
Syphilis is growing rapidly and especially among men who have sex with men (MSM). However, rising incidence rates are also being observed across all groups due to a relaxation in the use of condoms.
Switching to HIV, there are 32 million people infected who are alive. Mortality now has gone down thanks to antiretroviral therapy, but this is not the case in Africa or some parts of Asia, where there’s still a 50% mortality. PrEP has lowered the risk of HIV infection in 90%.
Lymphogranuloma venereum (caused by C. trachomatis L1, L2, L3) also had a rise among MSM.
Mpox is produced by a Poxviridae, typically an animal disease but spread among humans, especially MSM. They present with a smallpox-like rash, conjunctivitis… as well as the typical varioliform lesions on contact areas. There are some treatments available (tecovirimat, cidofovir, brincidofovir) but basically what’s needed is to wait until it resolves. There are also 2 vaccines which work moderately well: Jynneos, Acam2000
Measles: fever, diarrhoea, rash, Koplik spots (not always present but almost pathognomonic). It is still very rare, but the numbers are going up, especially in Africa, Russia, Brazil… due to people not being vaccinated. For the treatment, there’s a role for vitamin A (50-200k IU/day). But here the important issue is to get the MMR vaccine since it is a cause of preventable death in children <5.
Dengue is caused by an RNA flavivirus, and the vector is the Aedes mosquito. The problem is that with climate change these mosquitoes are becoming more widespread, with the incidence growing since 1960. The clinical presentation can be mild, so the patient doesn’t know they had it, but when present it’s a macular rash, myalgia, headache… but in 2% dengue can be haemorrhagic and can lead to death. There’s a vaccine now (live vaccine): Dengvaxia à 3 injections (0, 6, 12 months). It works but should be given only to people who have had dengue, otherwise there’s risk of have dengue with the vaccine.
Filovirus infections: Ebola. Luckily there’s less news about Ebola, basically because although fatality rate is 70-90%, there’s a highly effective vaccine for Ebola: Ervebo
Some new emerging mycoses include talaromycosis (in China and SE Asia), emergomycosis (in South Africa and South Europe), and Trichophyton indotineae (has received attention since the presentation resembles more psoriasis. Treat with itraconazole)
A new agent has been identified as a cause of leprosy, besides M. leprae: M. lepromatosis (found in 2008)
Atopic dermatitis: Latest advances and insights
Speaker: Dr. Bieber (Germany)
There are different subsets of patients in AD when we put them in biologic drugs: non-responders, partial responders, responders who have some ups and downs, and super-responders. This scenario typically works for most patients but doesn’t work for all of them. So, what we must aim at is not disease control but disease modification.
Report written by Dr Oriol Yelamos (dermatologist, Spain)
Chairs: Dr. Clio Dessinioti (Greece), Prof. Dr. Falk Ochsendorf (Germany)
Speakers: Dr. Clio Dessinioti (Greece), Prof. Aleksandra Lesiak (Poland), Prof. Dr. Falk Ochsendorf (Germany), Prof. Julien Lambert (Belgium).
Special focus: Pre-pubertal population
Speaker: Dr. Dessinioti (Greece)
Acne can occur at any age, although it may mean different things. Neonatal acne is relatively frequent (20% of neonates) and it’s due to maternal androgens crossing the placenta, therefore resolves spontaneously. Infantile acne can happen until 1 year of age and it’s due to enlarged adrenals, which is normal until 1 year. Later, acne can reappear due to adrenarche, which happens normally around age 7 or 8. Therefore, what is not normal is to have acne between age 1 and 7. Acne occurring at this age may indicate an adrenal tumour that releases androgens, or another cause of hyperandrogenism so it’s necessary to refer to a paediatric endocrinologist (premature adrenarche, congenital adrenal hyperplasia, Cushing syndrome, adrenal tumours, precocious puberty, thyroid diseases).
McCune-Albright syndrome is associated with acne, precocious puberty, bone fibrous dysplasia and café-au-lait macules (CALM).
The most well-known acne is prepubertal acne, which is due to the activation of the adrenal glands. Adrenarche is associated with onset of acne, body hair, body smell and seborrhoea. This is normal in most cases but, if acne persists or is severe, it is important to exclude hyperandrogenism so it’s necessary to refer to a paediatric endocrinologist.
The treatment of acne in childhood depends on the severity and the age. Typically, topical combinations (retinoids +- BPO +- ATB) are first line if acne doesn’t improve alone. Tetracyclines should NOT BE USED in little children since it affects bone development, so wait until teeth are fully formed. Isotretinoin is not approved for children <12, but in severe cases may be used off label (bear in mind we use isotretinoin since the first day of life in children with ichthyoses).
Special focus: Adult women
Speaker: Dr. Lesiak (Poland)
20% of adult women may have some form of acne in adulthood, and the incidence is rising.
The aetiopathogenesis is mixed: genetic predisposition, C. acnes... but, in this subset of patients, hormones are very important.
In addition, adult women can be subject to some aggravating factors for acne: stress, sleep disorders, excessive use of cosmetics, damaged epidermal barrier, UV radiation, endocrine diseases, obesity, smoking.
Hormones can be altered at different points in adult women acne, at the ovaries (being the most common one PCOS) but also it can be due to an increased sensitivity to hormones on the skin.
Also, these patients have changes in the sebum, with a decrease in linoleic acid, squalene peroxidation by C. acnes and UV, and an increase in free fatty acids. This all leads to epithelial destruction, hyperkeratinisation and inflammation.
Diet also is a hot topic, and it seems high glycaemic diets are associated with acne. This happens due to insulin resistance and an increase of adiponectin.
Some medications also induce acne, so we must ask our patients if they take something, paying attention to contraceptives, since the ones with levonorgestrel and other pro-androgenic contraceptives may worsen acne.
Stress increases cortisol and cortisol can induce acne.
Smoking also can induce acne due to nicotine effect on hyperkeratinisation.
How do we treat acne in adult women? Similar to other acnes, the most complete treatment family is retinoids +- BPO +- antibiotics. Topical azelaic acid also seems a good option, especially for pregnant women. When it’s not enough, we then use systemic treatments (tetracyclines, isotretinoin, spironolactone, antiandrogenic contraceptives).
Severe forms of acne
Speaker: Dr. Ochsendorf (Germany)
First, we must define what we think “severe acne” is, since there’s not really a consensus. There are several issues to take into the account regarding the clinical presentation: the extent, the lesion characteristics, and the presence of scarring. There are some classic forms we consider severe: acne conglobata, acne fulminans… We also must consider the psychological impact on the patient, and we need to know for how long the lesions have been present and the previous treatments.
Some cases of acne fulminans may be triggered by isotretinoin. Although about 30% of cases in which we administer isotretinoin for acne, only 4% may be severe. Which are the risk factors of having a severe flare of acne due to isotretinoin? They are more frequent in young males, >44 comedones, 2 nodules on the face, nodules on the trunk, global acne grading system >28.
What to do with these flares? Systemic corticosteroids for 2 weeks and lower isotretinoin dose.
When we face patients with nodules, we don’t have to drain it or cut them, since we will leave a scar. We need to inject small amounts of triamcinolone.
In severe cases and isotretinoin contraindication we can use dapsone 100mg/d, tetracyclines, injected triamcinolone or some light devices (IPL for example).
In refractory acne (when it doesn’t improve to treatment), the first thing you must do is make sure the patient is taking the medication correctly. If the patient is adherent, then you need to think if the treatment is correct (if the patient is taking antibiotics, maybe switch the patient to isotretinoin). If it still doesn’t improve (for example sandpaper acne), what you probably need to do is to wait (sandpaper acne works well with isotretinoin but requires time, >6 months).
Mimickers of acne
Speaker: Dr. Lambert (Belgium)
Acne is related with hidradenitis suppurativa (HS patients have a fourfold risk of having acne), but sometimes some lesions seem the same and they are not. In acne you find closed comedones, and HS has open comedones.
Also, you must suspect HS and not acne if you see involvement of the neck, retroarticular involvement, buttocks, under the chin, lumbar lesions, and/or boxcars/rope-like scars.
Knowing if it is acne or HS is relevant, since isotretinoin will not work in HS and may make the disease worse.
Another disease that is a mimicker of acne is lupus miliaris disseminates faciei. This presents with asymptomatic papules and nodules brownish to yellowish, typically on the central part of the face and on or around the eyelids. There are numerous treatments, meaning none work very well, and the best seems to be PDL laser.
Another mimicker is comedogenic lupus. It is an uncommon form of lupus. Lesions tend to be on the auricular region. Concomitant lesions of lupus help with the diagnosis.
Another acne mimicker that is frequent is Malassezia folliculitis. Pruritic papules in males on the upper torso and face. Recently, fluorescence dermoscopy has been suggested to be helpful to diagnose it.
Some drugs induce acneiform lesions, such as JAK inhibitors, what is call JAKne. Typically, it is mild and has no hormonal influence, so you can treat it as classic “teenage acne”: topical combinations, oral antibiotics, adequate skin care… JAKne is more common in JAK1 inhibitors (abrocitinib, upadacitinib), followed by JAK1+2 inhibitors (baricitinib), followed by TYK2 inhibitors (deucracitinib), and the least frequent ones producing acne are panJAK inhibitors (tofacitinib).
It is important to mention that some cases of more inflammatory lesions like rosacea appear with deucracitinib, and this more severe forms appear in skin of colour patients.
Report written by Dr Anna Zalewska-Janowska (Poland)
Chairs: Pr. Selim Aractingi (France), Dr. Shyam Verma (India)
Speakers: Pr. Selim Aractingi (France), Dr. Shyam Verma (India), Pr. Dr. Dr. Ralf Paus (Miami, USA), Pr. Dr. Emilie Sbidian (Créteil, France)
Steroids and the skin: From Cushing syndrome to Addison's disease
Speaker: Pr. Selim Aractingi (France)
Professor Aractingi presented a lecture on the ever-relevant topic of steroids and the skin — from Cushing syndrome to Addison’s disease. It is extremely important to thoroughly review textbook information on the key role of endocrinology in the presentation of skin diseases. Such revision should be practiced by every dermatologist. We should all remember that the skin is a powerful mirror of systemic diseases.
The consequences of cortisol on normal skin, such as the reduction of collagen I and III, were illustrated, along with the effects of increased cortisol signaling — telangiectasia, thinning, shininess, loss of skin markings, and sometimes striae. Cortisol binds to both glucocorticoid and mineralocorticoid receptors (MRs). The SPIREPI clinical trial on topical administration of the MR antagonist spironolactone showed that it can limit glucocorticoid-induced skin atrophy in healthy volunteers and improve wound healing.
Facial acanthosis nigricans
Speaker: Dr. Shyam Verma (Vadodara, India)
Dr Verma presented a very clinically oriented topic on facial acanthosis nigricans (FAN) which was very rich in clinical photographs. The presenter kindly allowed the audience to take pictures of the most important summary information on his slides that could help in expanding knowledge on facial acanthosis nigricans. FAN accounts for about 7.5% of facial melanosis predominantly in dark skin types (South Africans, Hispanics, Africans) in patients who are overweight or obese. In international literature FAN is rarely mentioned. This entity lacks precise definition of its clinical and histopathologic features. Clinical pictures were presented. As for investigations blood pressure measurements, serum lipid profile, blood glucose, blood insulin, thyroid function tests, serum uric acid, cardiovascular risk assessment, and polysomnographic sleep study should be performed. As for management – lifestyle modifications with weight reduction being of key importance, sunscreen use, chemical peels, topical retinoids and vitamin D analogues, systemic retinoids and insulin sensitizers, lasers were mentioned.
Skin-thyroid link
Speaker: Pr. Dr. Dr. Ralf Paus (Miami, USA)
The third lecture of the session was delivered by Professor Ralf Paus from Miami in the USA on skin-thyroid link. Human scalp hair follicles (HFs) are major neuroendocrine organs. HFs have a peripheral equivalent of the central hypothalamic-pituitary-adrenal (HPA) axis: CRH→ACTH →cortisol. HFs have a functional hypothalamic-pituitary-somatotropic (HPS) axis equivalent: GHRH, SST→GH→IGF-1. New ongoing issue is HFs PT axis TRH→TSH. Thyroid-skin connection: clinical perspectives are the following: short-term topical T3+TRH→ could be a novel strategy for managing telogen effluvium.
Obesity and the skin
Speaker: Prof. Dr. Emilie Sbidian (Créteil, France)
The final lecture was delivered by Professor Emilie Sbidian from Creteil in France on “Obesity and the skin.” The speaker pointed at obesity and being overweight as an important game-changer due to skin anatomy and physiological alterations in such states. Of importance, 97% of patients after metabolic and bariatric surgery (MBS) present resolution or mild form of their psoriasis. After MBS, most patients (78%) continued their psoriasis treatment, but a significant downregulation in medication category was noted, e.g., from systemic to topical agents. Therapeutic efficacy is influenced by obesity in many treatments. Another example is hidradenitis suppurativa. Take home messages from this lecture are the following: 1. Skin anatomy and physiology changes in obesity leading to the development of various skin manifestations and disorders; 2. Specific skin involvement as plantes keratodermas, striae distensae, skin infections, hyperandrogenism or insulin-resistance manifestations; 3. In the obese population, higher risk of chronic inflammatory skin disorders, higher severity, less efficacy of systemic treatments is observed; 4. In the obese population, adaptation of the treatment doses should be carefully performed; 5. Of note, we should be aware of skin side-effects of anti-obesity treatments.
Report written by Dr Anna Zalewska-Janowska (Poland)
Chairs: Pr. Dr. Martin Metz (Berlin, Germany), Pr. Margarida Gonçalo (Coimbra, Portugal)
Speakers: Pr. Dr. Martin Metz (Berlin, Germany), Pr. Margarida Gonçalo (Coimbra, Portugal), Pr. Dr. Mojca Bizjak (Golnik, Slovenia), Pr. Ana Giménez-Arnau (Barcelona, Spain)
Understanding chronic spontaneous urticaria
Speaker: Pr. Dr. Martin Metz (Berlin, Germany)
Professor Martin Metz delivered a lecture on “Understanding chronic spontaneous urticaria (CSU)”. The speaker started his lecture from the old reflection – what we did know about pathophysiology of urticaria 22 years ago? The conclusion was “not too much” – namely most of CSU patients had no effective treatment and CSU patients were among the most disliked patents by dermatologists. As for current hot info we can say that although CSU is not an allergy, total IgE is increased in most patients. Of importance more than 30% of CSU patients have IgG anti-TPO antibodies vs about 5% in the general population. Professor Metz pointed to the first published phase 3 study with omalizumab in CSU by Maurer at all in-N Engl J Med in 2013 that was the breaking through approach to CSU treatment ever. The speaker reminded the audience of autoimmunity type I (autoallergy) and type IIb in CSU. He also underlined the importance of modulators in CSU course including stress (neuropeptide release), foodstuffs (pseudo allergens), drugs (complement activation). Infections (bacteria activity). Next, the speaker focused on Mas-related G-protein receptor X2 (MRGPRX2) and its role in CSU. X-2 and IgE-mediated mast cell (MC) degranulation is additive, X2 is exclusively expressed by skin MC, targeting eosinophils (by anti-IL-5) can be effective in CSU and finally X2 agonists (i.e., substance P) are elevated in CSU. Next, the speaker concluded his lecture with the following statement: CSU is a debilitating disease that requires optimal treatment, MC is central pathophysiology of the disease, auto-IgE is a relevant mechanism in CSU and better understanding of CSU by novel-targeted treatment (KIT, BTK, MRGPRX2…) is highly desired. Then the speaker pointed to the legacy of Marcus Maurer who created an international network of Urticaria Centers (UCAREs), Chronic Urticaria Registry (CURE) – a global registry that collects real world data on patients with CSU, CIndU and urticarial vasculitis (UV) (www.urticaria-registry.com), and CRUSE control (ChRonic Urticaria Self-Evaluation – application of self-evaluation of urticaria status by the patients. Professor Metz finished his invaluable talk with great thanks expressed to Professor Marcus Maurer who tragically passed away last summer when he was enjoying his holidays in the Alps. This talk was very touching and motivating to all urticariologists!
Understanding chronic inducible urticaria
Speaker: Pr. Dr. Mojca Bizjak (Golnik, Slovenia)
Professor Mojca Bizjak from Golnik in Slovenia acquainted the audience with “Understanding chronic inducible urticaria.” The speaker pointed out that inducible urticaria (CIndU) affects up to 1.5% of global population and often co-exist with CSU. Professor Bizjak clearly demonstrated the subtypes of CIndU and diagnostic approach to each of the subtypes. The most frequent subtypes are symptomatic dermographism (CD), cold urticaria (coldU) and cholinergic urticaria (CholU). Diagnostic approach to CIndU is in depth presented in Allergy in 2024 (“An algorithm for the diagnosis and treatment of chronic inducible urticaria”). Provocation testing methods were clearly presented in one table with detailed instructions on how to test symptomatic dermographism (SD) on forearm or upper back. Of importance, 1/3 of cold urticaria patients can experience anaphylaxis! The speaker also pointed to novel biomarkers such as gut microbiome (Exp Dermatol 2021 – Identification of gut microbiome signatures in symptomatic dermographism) or miR-126-3p and miR-16-5p as novel serum biomarkers for disease activity and treatment response in SD (Clin Immunol 2021). The speaker also pointed to the observation that eating increases and exercise decreases disease activity in patients with symptomatic dermographism (J Allerg Clin Immunol Pract 2023). The speaker also summarized features of typical ColdU patients with previous anaphylaxis namely reactivity of the oral cavity or throat to cold was noted in about 1/3 of the patients, provocation test demonstrated shorter CSTT (critical stimulation time thresholds), pseudopodia on the edges of the main wheals, larger wheal diameter, higher CTT – critical temperature threshold. The speaker also demonstrated highly informative clinical photographs with skin reactions patterns in cholinergic urticaria (Allergy 2025). For those interested in mastering skin testing procedures in contact urticaria professor Bizjac recommended “Contact Urticaria and related conditions” clinical review published in Contact Dermatitis in 2025. As for emerging therapies the speaker pointed to barzolvolimab (completed phase 1) and remibrutinib (phase 3 – not completed).
Diagnostic workup
Speaker: Pr. Margarida Gonçalo (Coimbra, Portugal)
The third lecture of the session was delivered by Professor Margarida Gonḉalo on “Diagnostic workup” in chronic urticaria (CU). The speaker pointed to the following steps in CU workup: 1. Diagnosis of CSU – differential! 2. Severity evaluation and impact on quality of life (PROMs, CRUSE) 3. Characterization of CSU subtype (aaCSU/aiCSU), 4. prediction of treatment response (H1/C4/OMZ). In CIndU – confirmation of trigger threshold should be definitively performed. Diagnosis is clinical, lab tests include CBC, CRP, ESR, IgE. If there is no itch, atypical more persistent lesions with bruising, excoriations and systemic symptoms (fever/arthralgia) always perform differential diagnosis and look for autoinflammatory syndrome, urticaria vasculitis, bullous pemphigoid, urticarial dermatitis, or angioedema (bradykinin-mediated). Of importance, in differential diagnosis of severe CSU course consider normocomplementemic urticaria vasculitis. Differential diagnosis between urticarial vasculitis and CSU was published in Clin Trans Allergy in 2023 and deserves attention. Also, publication of Rothermel at all in Am J Clin Dermatol in 2025 on managing of urticarial vasculitis is worth being acquainted with. Autoinflammatory syndrome should be considered in differential diagnosis of CSU, including Sill’s disease (ferritin check), Schnitzler syndrome, bullous pemphigoid, urticarial dermatitis (good response to dupilumab), neutrophilic urticaria (histopathology exam indispensable), cryopyrinopathies (in children). In the diagnostic process of CSU apart from clinical picture the following lab exams should be considered” skin biopsy (DIF), CRP/ESR, ferritin, C3/C4, serum electrophoresis. Evaluation of disease severity and quality of life is particularly important (Sousa-Pinto B et al Allergy 2025). Biomarkers of anti-H1 poor response are angioedema, high USA7, long CSU duration, concomitant CIndU, high CRP, high D-dimers, high C3/C4/C5, presence of anti-TPO, very high IgE levels. Type IIb autoimmunity most probably can be discovered in mostly female, older patients with more severe CSU, more often angioedema, night symptoms, urticaria encountered in more than 5 days a week, longer CSU duration, slow or non-responders to OMA, low/very low IgE, on cyclosporin A (3mg/kg/day) for 2-3 months leads to complete and fast response with long remissions (when recurrence – still good response to CyA). Autoallergy in CSU and autoimmunity type II can overlap 10% of patients. Biomarkers for poor response to OMA are low IgE levels, selective IgE deficiency, low/absent FcεRI on basophiles, basopenia/eosinopenia, lower levels of IL-31, high body mass index (>30kg/m2), low OMA serum levels. The speaker concluded with Prof. Marcus Maurer’s motto: “Never give up!” and pointed at unmet needs including search for better biomarkers for severity of CSU endotypes (aa/ai CSU), predictors of response to OMA/CyA/Anti-H1 and dupilumab/BTKs/barzolvolimab. Personalized medicine in CSU and disease modification can lead to CSU cure and let’ s aim at that!
New treatments
Speaker: Pr. Ana Giménez-Arnau (Barcelona, Spain)
Professor Ana Giménez-Arnau from Barcelona in Spain presented unmet needs and emerging therapies in chronic urticaria. The speaker emphasized the necessity of treating the disease until it is resolved, highlighting that a complete response to treatment is associated with the least impact on health-related quality of life in patients with CSU. Consensus on CSU treatment was published in Allergy 2022 by Zuberbier et al (already new edition is accepted for publication in Allergy). Omalizumab was the first biologic treatment for CSU. This drug avoids IgE interactions with IgE receptor on mast cells (MC) and basophils, downregulates receptor expression on their surfaces, inhibits histamine release and does not bind IgE receptor and thus does not induce anaphylaxis. The speaker noted that IgE (cut-off≤ 43.8 IU/ml) could be a useful biomarker to predict the autoimmune diseases in CSU. She also underlined that antihistamine-resistant CSU remains undertreated. Next the speaker presented a rich table with novel treatments, targets, and trials. Currently of interest is remibrutinib – oral, highly-selective Bruton’s kinase (BTK) inhibitor which blocks the activation of mast cells (MCs) and basophils by blocking BTK. Remibrutinib is a covalent irreversible BTK inhibitor. It prevents MCs degranulation and subsequent development of CSU symptoms, and this effect is independent of the FcεRI activation. Remibrutinib causes a rapid improvement in UAS7 already in the first week of treatment which is maintained up to week 12 or even 24. Of importance, complete response (UAS7=0) was observed over 52 weeks. Rilzabrutinib is another BTK inhibitor to be investigated whereas fenebrutinib was stopped. Dupilumab in CSU is currently in phase 3 studies. The speaker pointed to a new consensus in CSU which is accepted for publication with second line treatments: omalizumab (already labelled), dupilumab (when labelled) and remibrutinib (when labelled). Third line therapy denotes cyclosporine A add on to second line. Barzolvolimab is another option in CSU treatment. It is an anti-KIT monoclonal antibody aiming at MCs depletion. Barzolvolimab selectively inhibits stem cell factor (SCF) dependent KIT activation by binding to the extracellular domain of KIT receptor thus inhibiting interaction with SCF. This drug leads to significant decrease in CSU activity and is well tolerated (phase 2 trial). Of importance, patients with prior OMA experience have similar prolonged improvement in UAS7 for at least 28 weeks after the last dose. Another option seems to be MRGPX2 antibody which is phase 2 CSU trial and open label study for cold urticaria. However, for clinical practice, the management of CSU should involve close cooperation between patient and physician. We should always focus on quality of life of our patients!
Report written by Dr Anna Zalewska-Janowska (Poland)
Chairs: Pr. Elke Weisshaar (Heidelberg, Germany), Pr. Laurent Misery (Brest, France)
Speakers : Pr. Laurent Misery (Brest, France), Pr. Elke Weisshaar (Heidelberg, Germany), Pr. Bernhard Homey (Düsseldorf, Germany), Dr. Emilie Brenaut (Brest, France)
Neurosensitisation in itch
Speaker: Pr. Laurent Misery (Brest, France)
The first lecture was delivered by professor Misery on Neurosensitisation and itch exploring those mechanisms in depth.
Immune mechanisms of itch
Speaker: Pr. Bernhard Homey (Düsseldorf, Germany)
The second lecture was presented by Professor Bernhard Homey from Düsseldorf in Germany entitled “Immune mechanisms of itch.” The speaker acquainted the audience with the complicated mechanisms of itch pointing at the finding that IL-31 (currently regarded as the main interleukin of itch) stimulation of human basophiles induce a distinct transcriptome-bridging type 2 responses towards type 3 inflammation modules. The speaker pointed out that pathways induced by IL-31 in basophiles are downregulated by IL-31RA inhibition in atopic dermatitis (AD) and prurigo nodularis (PN) patients. What is more, PN transcriptomic signatures overlap with both AD and psoriasis. Data suggests that PN is not a classical disease but also associated with IL-17 signaling. In PN abnormal – increased keratinocyte differentiation, mixed immune signaling pathways are observed. What is interesting IL-31 is activating human dorsal root ganglion (DRG) neurons and induces a scratching behavior in animals (monkeys, mice, dogs). IL-31 induces itch and sensitizes sensory neurons to other pruritogens (pretreatment with IL-31 enhanced itch induced by the pruritogen – histamine, LTC4, clozapine-N-oxide and chloroquine). IL-31 promotes neuronal growth – total DGRs, cultures DGR, DGR explants). IL-31 induces neuronal growth, nerve elongation, and branching. In OLYMPIA 2 study PN patients were treated with nemolizumab and significant improvement in itch and skin lesions was observed (spectacular clinical photos presented). Sleep disturbance decreased during weeks 4 and 16. In conclusion, prof. Homey stressed that the itch-scratch inflammation cycle represents a key principle in the pathogenesis of AD and PN. Additionally, multiple mediators and effector cells drive this cycle through a variety of pathways, leading to increased itch, inflammation, and epidermal dysregulation. And finally, IL-31 pathway has an important effect on inducing and amplifying the activation of pruritogenic neurons.
Pruritus of unknown origin workup
Speaker: Pr. Elke Weisshaar (Heidelberg, Germany)
Professor Weishaar delivered the third lecture of the session and acquainted the audience within depth workup of chronic pruritus of unknown origin (CPUO). First, the speaker pointed out CPUO characterization issues. CPUO is the entity where no identifiable etiology can be determined, no identifiable cause can be elucidated. Of note, no identifiable cause can be determined after a thorough investigation and check-up. Characterization of CPUO based on retrospective cohort study of 263 CPUO patients presenting to a specialized pruritus center presents as follows: pruritus lasting longer than 1 year (77.6%), occurring daily (68.2%), occurring in attacks (72.8%), was accompanied by burning, tingling and stinging sensations (79,5%), was reported with the worst itch intensity ≥ 7 on the VAS in the previous 4 weeks (58.8). Clinical trial (LIBERTY-CPUO-CHIC) study has recently started and is based on subcutaneous administration of dupilumab in adult CPO patients. The speaker also pointed to the European guidelines on chronic pruritus published this year (Weishaar et al, Acta Derm Venereol 2025).
Management of itch
Speaker: Dr. Emilie Brenaut (Brest, France)
Finally, dr Emilie Brenaut from Brest in France delivered a lecture on “Management of itch.” New definition of pruritus based on a multidisciplinary Delphi consensus (on the modern definition of pruritus) is presented in the JEADV in 2025 by Ständer et al. .Readers interested in widening the knowledge in the subject could get them from the publication entitled “European Guideline on Chronic Pruritus in cooperation with the European Dermatology Forum (EDF)” by Weisshaar et al (Acta Derm Venereol 2025). The speaker underlined the general measures that could be applied including: soft clothing permeable to air e.g. cotton and dress in layers to avoid sweating; low room temperature at night; mild, non-alkaloid perfume-free soaps, moisturizing syndets and shower/bath oils; skin moisturizer on a daily basis, especially after showering or bathing; emollients, especially at night: cream/lotions/gels with e.g. urea (5-10%), glycerol (20%), camphor (2%), menthol (1%), zinc (10%), pramoxine (1%), polidocanol (2-10%); luke-warm water; bath (max 20 minutes); possible adding oatmeal or potassium permanganate; dabbing the skin after bathing; cooly wet or fat-moist wraps. Dr Brenaut pointed also at the necessity of avoidance of such factors as excitement, strain, negative stress; very hot and spicy foods, large amounts of hot drinks and alcohol; contact with allergenic and irritant substances such as fragrances, preservatives; factors that can contribute to dry skin, such as dry climate, sauna, alcoholic compresses, frequent washing and bathing. Relaxation techniques and education training programs for coping with vicious itch-scratch cycle were also pointed at (Weisshaar et al, Acta Derm Venereol 2019). The speaker also recommended publication by Ständer et al on prurigo guidelines (Itch 2020) where 4 steps of management are presented. Step 1 consists of topical corticosteroids, topical calcineurin inhibitors and H1-antihistamines. In step 2 the following management is delivered: topical capsaicin, intralesional corticosteroids, UV phototherapy. In step 3 gabapentin, pregabalin, antidepressants, cyclosporine and methotrexate are used. Step 4 consists of the following therapeutics- NK1R antagonist, µ-opioid receptor antagonist, dupilumab, nemolizumab. Of course, JAK inhibitors are recommended in selected cases of severe refractory prurigo in patients younger than 65 years. The following drugs are in trails: barzolvolimab (KIT antagonist), povorcitinib (JAK 1 inhibitor), Rocatinlimab (OX-40 antagonist), vixarelimab (OSMRβ antagonist) and ruxolitinib cream (JAK1/2 inhibitor). The speaker concluded that identification and treatment of the cause of itch is of prime importance. Yes - definitely!
Report written by Dr Anna Zalewska-Janowska (Poland)
Chairs : Pr. Dr. Carmen Maria Salvastru (Bucarest, Romania), Pr. Dr. Georg Stary
Speakers: Dr. Lucinda Claire Fuller (London, United Kingdom), Pr. Ramon Grimalt (Barcelona, Spain), Dr. Martin Kassir (Dallas, United States)
New ways of approaching global skin health
Speaker: Dr. Lucinda Claire Fuller (London, United Kingdom)
The first speaker of the session – dr Lucinda Claire Fuller from London in the UK presented her lecture on the “New ways of approaching global skin health.” The aim of the whole dermatological society worldwide should be to leverage the impact and influence of dermatology in the international health area. Global health dermatology improves: 1. skin health worldwide (better healthcare, easier access); 2. research, teaching, and clinical practice; 3. social and political determinants of health and 4. focus on areas of resources limitations. Dr Fuler acquainted the audience with international bodies that take care of global health. Dermatology at World Health Organization (WHO) is focused on neglected tropical disease (NTD), noncommunicable diseases, healthy ageing in life course, occupational health, essential medicines, international classification of diseases (ICD-11), migrant health dermatology, emerging infections, environment, climate change and health (chemical safety). According to the Universal Health Coverage (UHC) and 3rd United Nations sustainable development goals 2015, all individuals and communities receive the health services they need without suffering financial hardship. Full spectrum of essential, quality health services, from health promotion to prevention (treatment, rehabilitation, palliative care) should be delivered throughout the life course. To me it is an ideal approach, but one question should be asked “Who is going to pay for that?” Neither this question nor answer to it I heard during the lecture, but it is crucial to have goals and dreams, especially humanistically oriented! Going back to the lecture, let us focus on the requirements to effectively address skin diseases in the context of UHC. We need clinical skill trained people, treatment, information, financial investment and the most important to me – political will and policies. The speaker pointed at establishing the Regional Dermatology Training Centre in Tanzania (established in 1992) and Pacific Regional Dermatology Training Centre which have been serving and training specialists for the regions. The following books were issued: Global Psoriasis Atlas, Global Atopic Dermatitis Atlas, Global Hidradenitis Suppurativa Atlas and Global Vitiligo Atlas. The speaker pointed to the huge importance of patient-reported impact of dermatological disease (PRIDD) which is the only fully validated dermatological impact measure applicable to all skin conditions (globalskin.org). It consists of 16 questions, and they can be answered in less than 2 minutes (Acta Derm Venereol 2024; 38 (7), 1391-1440.) Of vast importance is the fact that 78th World Health Assembly (WHA) on 24th May 2025 endorsed resolution on “Skin diseases as a global public health priority” which is essential for Universal Health Coverage. WHA is a decision-making body with 194 members states, which has annual meeting in May. WHA sets priorities and policies on health goals and health strategies and reviews reports on previous implementation areas to obtain better health and wellbeing for all. WHA resolution sets broad policy, requests action, creates political commitment, fosters accountability, sets precedent, executes highest level of commitment, guides national health system work, influences WHO program at work. Next, Dr. Fuller focused on WHO (World Health Organization) which is United Nations (UN) specialized health agency, which connects nations, partners, and communities. WHO promotes highest attainable health standards for all regardless of race, religion, gender, political belief, economic and social condition. WHO lead the world’s response to health emergencies, preventing disease, root cause of health issues, access to medicines and health care. WHA resolution can achieve the following goals on skin diseases: prioritize all skin diseases, strengthen surveillance and data collection, upskill primary health care, improve access to treatment, encourage integrated approaches (integrate skin health services with other programs e.g. NCDs, child health, mental health, stigma reduction initiatives) and prioritize perspectives of patients and their caregivers in all aspects of planning and implementation. Key aspects of WHA resolution are formation of the framework on “Global Action Plan” on investment, research, surveillance and data collection, equitable access, and integrated services. 78th World Health Assembly called upon 194 Member States to strengthen dermatology capacity at primary healthcare, increase access to affordable diagnostics and medicines, invest in workforce, and reduce stigma. 78th WHA also calls upon international organizations and partners to support national implementation, foster cross-sector collaboration and mobilize resources. WHO is called to develop a Global Plan of Action of Skin Diseases in consultation with Members States and stakeholders for consideration at WHA 80 in 2027. Another international body, i.e., WHARI (World Health Assembly Resolution Implementation) Task Force focused on advocacy and communication, resource mobilization, policy and country implementation, research and civil society and patients’ groups. Next Dr. Fuller presented different activities prepared for the World Skin Health Day 2025 which is celebrated on 8th July. The speaker also presented another good news that WHO Essential Medicines List was issued in September 2025 and “Section 13 Dermatological Medicines” contains 13.4. medicines affecting skin differentiation and proliferation (ustekinumab and adalimumab for psoriasis), 13.6 Moisturizes (5% urea and 15-20% glycerol regulatory approval as emollients for atopic dermatitis), 13.7. Sunscreen, broad spectrum for prevention of skin cancer in persons with albinism). It is advisable to get acquainted with the full text of the Skin Diseases Resolution.
5-alpha reductase inhibitors “Facts and Myths”
Speaker: Pr. Ramon Grimalt (Barcelona, Spain)
The next lecture was delivered by Professor Ramon Grimalt from Barcelona in Spain on 5-alpha reductase inhibitors – “facts and myths”. This extremely interesting talk was focused on the so-called post-finasteride syndrome (PFS). This entity has gained little attention in scientific world whereas google search revealed more than 4,900,000 results. PFS gained attention in 2012 when Dr Irwing, an endocrinologist from John Washington University, described depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects (J Clin Psychiatry 2012;73:1220-3). This publication was based on personal experience of dr Irving of 5 to 10 patients complaints. He also wanted MERK to label Propecia with the warning of persistent sexual side effects on its label. Publications that followed this first report were mostly published in non-dermatological, low-impact journals and most of them stated that there is no evidence that finasteride caused PFS. Current research also confirms this conclusion and puts emphasis on patient education and the need of thorough risk assessment when prescribing finasteride to males suffering from androgenic alopecia. Rivetti et al (J Cosmetic Derm 2025) conclude that the so called PFS may reflect a “pre-existing cognitive vulnerability” being a reaction to a widely stigmatized medication. Such individuals should never be prescribed finasteride. The researchers suggest that validated questionnaires such as Whiteley Index (designed to evaluate health-related anxiety) and Body Dysmorphic Disorder Questionnaire (BDDQ, used to screen for body image disturbance) should be used before prescribing finasteride. Those questionnaires could help identify patients who may benefit from enhanced counseling or psychological referral before initiating finasteride therapy. Gupta et al (2025) did not reveal any significant correlation between finasteride and depression/suicide reports from 2006 to 2011 but noted considerable number of such reports in 2013-2018 and 2019-2023. This increase could be attributed to heightened awareness of adverse events following recognition of the so-called PFS in 2012. Of note, special foundation was created to gather people from so-called PFS and collect resources to conduct so-called research in this field. The speaker finished his most engaging lecture with the set of very practical conclusions: 1. only highly concerned patients attend a dermatologist for androgenic alopecia treatment; 2. those concerned male tend to be more observant; 3. any health problem sexual or not that might appear during any treatment and might erroneously be attributed to the drug; 4. obsessed male tend to “notice” with more detail; 5. web pages and journalists not always act adequately; 5. practitioners should be extremely careful in selecting patients for finasteride treatment and be understandable to the fears of their patients; 6. Use of new drugs and formulations that are not easily spotted on the web is advised. So, final conclusion of this magnificent lecture – to me – is “everything is in our heads”. Let’s not allow nocebo effect to take over our life and function!
Robotics in dermatology
Speaker: Dr. Martin Kassir (Dallas, United States)
The final lecture of the session was presented by dr Martin Kassir from Dallas in the USA on “Robotics in dermatology.” The speaker started to acquaint the audience with general view of robotics in surgery and its advantages: Aka robot assisted surgery, minimally invasive surgical technique, employment of specialized robotic systems assisting surgeons in performing procedures providing unparalleled precision and control. Dr Kassir gave historical perspective of the area pointing to 1985 as beginnings of robotic surgery namely PUMA560 that performed neurosurgical biopsies. In early 2000 the Vinci surgical system with multiple robotic arms and surgical instruments that allow surgeons to fully control that equipment from the operating room was launched. Robotic instruments are designed to replicate human hand’s range of motion and perform surgical tasks (suturing, dissecting cauterizing, cutting). These systems revolutionized minimally invasive surgery and provided surgeons with enhanced dexterity, precision, enabling intricate and delicate maneuvers, improved visualization (3D), reduce risk of errors, filter out tremors, eliminate unintended movements, improve patient outcomes, and reduce post-operative complications. Robotic systems vastly reduce surgeon’s fatigue. Professionals have seated positions; no prolonged standing is required thus professionals maintain peak performance. Robotic instruments are currently used in cholecystectomy, hernia surgery, appendectomy, hysterectomy, myomectomy, prostatectomy, coronary artery bypass surgery (CABG), mitral valve, knee and hip replacement, spine surgery. Transoral robotic surgery (TORS) is employed in head and neck cancers, difficult to reach areas leading to improved speed and swallowing. Next the speaker turned to dermatology and pointed to the following areas: precision surgery, hair replacement, laser delivery, imaging, and mapping and finally telepresence. In dermatology, robotics can augment clinician dexterity, repeatability/reproducibility, and imaging-guided precision. Dr Kassir gave an example of hair restoration where automated follicular harvesting, robot-assisted microdissection, robotic arms implementation for laser delivery and teleoperated devices for remote procedures were used. Currently the following types of robotics are used in dermatology: surgical robots for skin cancer removal and reconstruction, diagnostic robots for skin lesion analysis, automated laser systems for precise treatment, tele-dermatology robots enabling remote consultations. Dr Kassir pointed to the following applications of robotics in dermatology: early detection and diagnosis of skin cancers (e.g. melanoma) automated biopsy and microsurgery procedures, treatment of chronic skin conditions (e.g. psoriasis) using laser therapy, robotic assistance in cosmetic dermatology (e.g. Botox, fillers), enhancing accuracy and reducing human error in procedures. Of note, “diagnostic robots” for skin lesions analysis already exist in the form of AI-driven systems (artificial intelligence-driven), specialized devices, or use of AI to detect skin cancers or other skin diseases. The speaker gave few examples of tools such as 3D-whole body imaging systems (Vectra WB360) and AI-powered apps that analyze smartphone-captured dermoscopic images. Robotics can provide expert-level diagnostics in remote or primary care settings and help the health system to combat shortages of professional human force.
Report written by Dr Anna Zalewska-Janowska (Poland)
Chairs: Assoc. Pr. Beatrice Dyring-Andersen (Copenhagen, Denmark), Pr. Dr. Knut Schäkel (Heidelberg, Germany)
Speakers: Dr. PhD Alexandros Onoufriadis (Thessaloniki, Greece), Dr. Ravi Ramessur (London, United Kingdom), Pr. Dimitra Kiritsi (Thessaloniki, Greece), MD, PhD Kelsey van Straalen (Rotterdam, Netherlands)
Precision diagnostics for genodermatoses
Speaker: Dr. PhD Alexandros Onoufriadis (Thessaloniki, Greece)
Dr Alexandros Onoufriadis from Thessaloniki in Greece presented the lecture entitled “Precision diagnostics for genodermatoses.” The speaker pointed at lower cost of human genome analysis nowadays and easiness of purchasing the exam results by every Homo sapiens representative. Dr Onoufriadis acquainted the audience with NGS (next generation sequencing) as a method of lower cost, higher speed that reads even lengths from 35bp than other methods. He also pointed at the importance of WES (whole exome sequencing) even though only a small percentage of the human genome encodes proteins. Of note – exons harbor approximately 85% of the mutations with large effect on disease development. The speaker underlined that based on genetics – effective treatment can be introduced and gave example of generalized pustular psoriasis (GPP). Disease alleles associated with GPP have been identified subsequently in AP1S3 (encoding a subunit of the adaptor protein 1 complex) and CARD14 (encoding a keratinocyte nuclear factor kB adaptor protein). Of interest, interleukin 1 blockers (IL-1 blockers – anakinra) has been shown to cause initial rapid clinical improvements in case reports, although full disease remission was seldom achieved. Of importance, development of IL-36 blockers for GPP was very successful and resulted in spesolimab approval for GPP treatment (FDA approval in 2022). The speaker underlined the importance and usefulness of precision diagnostics for complex phenotypes where double gene pathology is encountered. Precision molecular diagnostics via NGS technology has improved the characterization and classification of inherited disorders, including genodermatoses. The speaker stressed that clinical diagnosis of inherited disorders may be challenging in consanguineous pedigrees particularly if more than one disease is present however, WES analysis is helpful and highly recommended in searching for proper diagnosis.
Beyond the plaque: Genetic insights into the link between psoriasis and its comorbidities
Speaker: Dr. Ravi Ramessur (London, United Kingdom)
The second lecture was delivered by dr Ravi Ramessur from London in the UK – “Beyond the plaque: genetic insights into the link between psoriasis and its comorbidities.” The speaker pointed at genetic testing as a helpful tool for guiding management in psoriasis patients due to genetics ability to discover disease subtypes, predict comorbidities for earlier intervention and finally clarify causal links with comorbid diseases. Of importance, genetics combined with other molecular data and advanced computational tools, moves us closer to personalized, more effective care in psoriasis. Dr Ravi underlined vast burden of comorbidities in psoriasis including psoriatic arthritis, obesity, increased level of LDL, triglycerides, metabolic syndrome, NAFLD, cardiovascular disease, hypertension, diabetes, and systemic inflammatory response. The speaker pointed at some aspects of psoriasis management in the future namely pro-active care based on prediction and primary prevention of disease development and targeting biological pathways linking psoriasis with comorbidities aimed at targeted treatment for psoriasis and/or comorbidities. Genome wide association analysis (GWAS) studies can reveal disease subtypes, prediction and causal relationships leading to targets for existing psoriasis therapies (Dand et al Nature Communications 2025). The speaker pointed at 200 genetic variants already discovered that best distinguished psoriatic arthritis from cutaneous form of the disease. Of importance machine learning models using these variants showed high accuracy (AUC around 0.82). Sounds fascinating!
Drug repurposing for genodermatoses: From understanding the pathogenesis to disease relief
Speaker : Pr. Dimitra Kiritsi (Thessaloniki, Greece)
Next, Professor Dimitra Kiritsi from Thessaloniki in Greece acquainted the audience with her presentation on “Drug repurposing for genodermatoses: from understanding the pathogenesis to disease itself.” The speaker presented very interesting examples how so-called old drugs could be helpful in genodermatoses when pathogenetic pathways of the diseases are revealed. Professor Kiritsi presented cholesterol biosynthesis pathway in congenital hemi dysplasia with ichtyosiform erythroderma and limb defects (CHILD syndrome) demonstrating that combination of statin with cholesterol normalizes skin structure in specific ichthyosis subtypes and is worth trying in clinical practice. It was also observed that inflammation is a disease driven factor in RDEB (recessive dystrophic epidermolysis bullosa) and losartan in children with RDEB in a phase I/II clinical trial (REFLECT) has already brought reliable results. Another example is secukinumab in Netherton syndrome (NS). Two patients with erythrodermic phenotype of NS showed marked improvement. NS subtypes share IL17/IL-36 signature with distinct type I IFN. Dupilumab for NS was also helpful. Note, enhanced expressions of IL-17-related genes and cytokines in the skin was observed in patients with Darier’s disease. Th17-associated cytokines i.e., IL-17 and IL-23 in inflamed skin in Darier’s disease are potential therapeutic targets. The speaker concluded that bench to bed site approaches have led to development of novel approaches for diverse types of genodermatoses but bed site to bench way is also feasible because animal models of the diseases are not always helpful in searching for effective therapies. Use of different approaches and combination therapy seems to be the best option, namely gene therapy combined with anti-inflammatory agents. However, as with everything, long-term side effects are to be elucidated first.
Advances in hidradenitis suppurativa
Speaker: MD, PhD Kelsey van Straalen (Rotterdam, Netherlands)
The final lecture of the session was delivered by dr Kelsey van Straalen from Rotterdam in Netherlands on “Advances in hidradenitis suppurativa.” First, the speaker pointed at topics on the subject from the last year, namely epidemiology of hidradenitis suppurativa (HS) that shows striking geo-variation, genetics achievements based on GWAS analysis, tunnel focused biology, microbiome as active part of the disease and finally anti-IL-17 therapies, anti-IL1 strategies re-emergence, JAK inhibitors moving from case-based evidence to randomized data. Of importance, global prevalence of HS is estimated to be 0.98%, but high regional variations are observed. In Bangladesh, Greece, Iran, and Sri Lanka – HS prevalence is equal or less than 0.3%, whereas in Nigeria, France, Saudi Arabia, or Greenland more or equal to 3%, everywhere female predominance is noted. Those variations implicate some undefined environmental factors and differences in genetic architecture. GWAS analysis demonstrated around 10 susceptibility loci of HS whereas the following 3 are the most common: SOX9 – transcription factor essential for hair follicle formation; KLF5 – transcription factor governing sphingolipid metabolism and barrier function in the skin; HLA – moderate effect size compared to classic auto-immune diseases. Tunnels observed on histopathology exams, earlier viewed as inert fibrotic end-stage features of HS, are now recognized as distinct microenvironments with cellular, molecular, and microbial components that perpetuate the disease. In conclusion, tunnels are regarded as active sources of inflammatory signals in HS. Around the tunnels the following cells are observed: neutrophils (neutrophil extracellular trap formation, response to microbes, amplification of inflammation); T cells; B and plasma cells (autoantibody production); tertiary lymphoid follicles (local communication without requiring circulation to draining lymphoid tissue). Next the speaker underlined the importance of tunnel microbiome. Namely polymicrobial microbiomes dominated by gram-negative anaerobic bacteria (Porphyromonas, Fusobacterium, Prevotella) is observed in tunnels, ready to form biofilms. Of importance, combination therapy both systemic and local targeting epithelial proliferation, immune response and microbiome should be introduced to HS patients.
Report written by Dr Anna Zalewska-Janowska (Poland)
Chairs : Pr. Marie-Aleth Richard (Marseille, France), Pr. Menno de Rie (Breukelen, Netherlands)
Speakers: Pr. Olivier Chosidow (Paris, France), MD, PhD Veronica Kinsler (London, United Kingdom), Pr. Dr. Markus Böhm (Münster, Germany)
What’s new in the treatment of scabies
Speaker: Pr. Olivier Chosidow (Paris, France)
The first lecture was delivered by Professor Olivier Chosidow from Paris in France on “What’s new in the treatment of scabies.” Perfectly clinically oriented lecture - necessary for every practitioner. 5% permethrin cream is the first line treatment in scabies, but only if good adherence/acceptability is obtained, and not in institutions and in the absence of molecular resistance. Otherwise, oral ivermectin is recommended, after available in countries topical scabicides have been tried without sufficient success. Also, oral treatment together with topical is recommended. In severe scabies ivermectin – 3 standard doses + 5% permethrin (2 applications+ emollients/keratolytics +/- hospitalization) is regarded as a minimal standard of care. This regimen can be increased/repeated in the most severe cases. The first randomized clinical trial ever performed in severe scabies revealed that ivermectin in high dose was not better than standard dose. Combination of 3 courses of oral ivermectin (day 0-day 7-day 14) together with 5% permethrin cream 2 applications (day 0 - day 7) leads to 75-80% of cure rate. This is a minimal standard of care to be currently used. In the future 3 closer courses of ivermectin (up to 7 courses) could be discussed. Topical scabicides could be applied every day or every other day, however this issue requires further clinical observation.
ESDR Plenary Lecture: Unique insights from mosaic disorders of oncogenes
Speaker: MD, PhD Veronica Kinsler (London, United Kingdom)
ESDR (European Society for Dermatological Research; www.esdr.org) plenary lecture was presented by Professor Veronica Kinsler and entitled “Unique insights from mosaic disorders of oncogenes.” The speaker started from an especially useful definition of what mosaic disorders are and illustrated them with a truly clear picture. Namely mosaic disorders are caused by single cell mutation during development, and they affect a part of the body. Prof. Kinsler published a highly informative article richly illustrated by clinical pictures entitled “Mosaic disorders affecting pigmentation – part 1: how to make a clinical diagnosis (Br J Dermatol 2025). The speaker underlined why mosaic disorders are important. Namely mosaic disorders have systemic health implications, carry malignancy risk, and can be passed to the offsprings. There are 3 developmental patterns of mosaicism: 1. Blaschkolinear/epidermal affected by the middle line; 2. Non segmental/ dermal not affected by the middle line; 3. Segmental/dermatomal, affected by the middle line. Prof. Kinsler pointed to the iOSAPP application (What is that Spot?) which will be on APP store soon and could help its users in clinical diagnosis. Of importance, melanocytic naevi share the same genetic causes – congenital or acquired. Mole reversal would be expected to reduce melanoma incidence. Of note, congenital melanocytic naevus (CMN) is the major risk factor for pediatric melanoma whereas number of acquired melanocytic naevi (AMN) is a strong risk factor for melanoma development – 20-30% of melanomas arise from an AMN. Mole reversal for melanoma prevention would be the first-in-man clinical trial. Professor Kinsler and her team are currently searching for financial support to perform this clinical trial.
René Touraine Plenary Lecture: The changing landscape of understanding and treating vitiligo.
Speaker: Dr. Markus Böhm
Final plenary lecture called René Touraine Plenary Lecture “The changing landscape of understanding and treating vitiligo” was delivered by Professor Markus Böhm from Münster in Germany. First the speaker acquainted the audience with the idea of René Touraine Plenary Lecture and Foundation (www.fondation-r-touraine.org). Global lifetime prevalence of vitiligo is estimated to be 0.36. Of note, more than 2,700,000 vitiligo patients are European inhabitants. There are following diagnostic and therapeutic gaps identified by global health care research in vitiligo: 1. the mean time until correct diagnosis is 2.4 years; 2. 44.9% of patients reported previous misdiagnosis; 3. many patients (56.7%) reported being told that vitiligo could not be treated!; 4. a quarter (25%) of healthcare professionals did not believe that an effective therapy for vitiligo exists; 5. 44.6% of patients reported giving up on finding an effective therapy (Bibeau et al J Eur Dermatol Venereol 2022; Hamzavi et al Br J Dermatol 2023). Of note, vitiligo is one of the top skin diseases that may lead to stigmatization and have increased rate of psychosocial comorbidities (3-25% of suicidal ideation and 3.3-3.7% suicidal attempt). Vitiligo seems to be regarded as a systemic inflammatory disease just like psoriasis or atopic dermatitis (Tulic et al JID 2024). Vitiligo diagnosis is quite easy to put forward but we should be aware of vitiligo-like conditions related to drugs (interferons, interleukin 2, imiquimod, checkpoint-inhibitors, alemtuzumab etc.). The speaker pointed at the differences between non-segmental vitiligo - NSV (genetic/familiar disposition, generally starts later in life, all body sites involved, associated with other somatic disorders, unpredictable course, relapses are common, significant impact on patients’ lives with psychosocial comorbidities, the first approved therapy is now available!) and segmental vitiligo - SV (sporadic, begins often in childhood, head, trunk and upper extremities are mostly involved, leukotrichia early, stops usually after 24 months, most often no relapse after therapy but no approved therapy exists so far, 10% may evolve into non-segmental vitiligo). Xu et al (Nature 2022) put forward a hypothesis that anatomically distinct fibroblasts subsets determine skin autoimmune patterns seem to be the explanation for the pattern of NSV development. Prof. Böhm put forward the following steps in NSV pathogenesis: genetic predisposition with 50 susceptibility loci, melanocytorrhagy (chronic melanocyte detachment) caused by trauma, friction, catecholamine, reactive oxygen species or autoimmune reaction, intrinsic abnormalities with increased melanocyte vulnerability in vitiligo – in quasi normal skin; altered E-cadherin expression/distribution in “normal” vitiligo skin; Th1 cytokine-mediated MMP-9 production and E-cadherin disruption. The speaker specially underlined shared decision making with the vitiligo patient. Such approach leads to better adherence and finally to increased therapeutic efficacy and improved quality of life of the patients. Of note, the treatment algorithm in NSV should be based on both stabilization and re-pigmentation pathways. Janus kinase (JAK) inhibitors were approved for NSV in April 2023 – for facial involvement in children from 12 years on and for adults – topical ruxolitinib (JAK1/2 inhibitor). The speaker presented very convincing clinical pictures from the real life of patients on ruxolitinib treatment. Of importance, systemic JAK inhibitors in NSV, namely upadacitinib and povorcitinib demonstrated reliable results in phase II trials. Other therapeutic ideas involve targeting: 1. skin resident memory T cells (phase I and II trials in progress); 2. melanocortin-1 receptor (phase III trial in progress); 3. neuropeptide calcitonin-gene-related peptide (CCGRP) -in a pilot study topical application of Rimegepant ointment alleviated skin depigmentation in patients with vitiligo.



EADV 2024 Bioderma Congress Reports