3 professionals
EADV 2024 Bioderma Congress Reports
EADV 2024 Bioderma Congress Reports
Get access to exclusive dermatological services to increase your professionnal knowledge: +500 pathology visuals, clinical cases, expert videos
Benefit from valuable features: audio listening, materials to be shared with your patients
Stay informed about the upcoming events and webinars, latest scientific publications and product innovations
Already have an account? login now
Reports written by Dr. Jovan Lalosevic (Dermatologist, Serbia), Dr. Nicolas Kluger (Dermatologist, Finland) and Dr. Stella Michelaki, M.D., Ph.D. (Dermatologist, Greece)
Related topics
Chairs: Prof. Kilian Eyerich and Dr. Hok Bing Thio.
Speakers: Prof. Kilian Eyerich, Dr. Hok Bing Thio, Prof. Hervé Bachelez, Dr. Satveer Mahil.
Report written by Dr Jovan Lalosevic, M.D., Ph.D.
Speaker: Prof. Kilian Eyerich (Freiburg)
In this updates session, the first lecture was given by Prof. Eyerich, who started with the definition of disease modification. In order to influence the course of the disease, we need to know the objective measurements that quantify clinically meaningful outcomes. In the case of psoriasis, the PASI score is not a valid way of measuring inflammatory activity.
Even if we could measure disease activity, we would then need a cut-off value to further define whether there has actually been success in modifying the course of the disease.
Referring to the expert opinion regarding the different definitions of disease control, remission and modification, he further stated that all experts can agree that the cure of a disease (psoriasis) is a maximum effort to modify a disease.
As factors such as race, gender and age do not influence the course of the disease, the only factor that does and that we can influence is the duration of the disease.
In the following lecture, Prof. Eyerich explained that introducing biologic therapy in the early stages of psoriasis can prevent the expression of systemic comorbidities, as it is well known that psoriatic arthritis and cardiovascular complications are part of the systemic spectrum that this disease manifests. By preventing comorbidities, we can actually modify the course of the disease.
It is postulated that this effect is produced by affecting the memory of resident cells in the tissue. In the skin, these cells are reduced over time when patients are treated with biologic treatments. The effect on these tissue memory resident cells is also present with other topical and systemic treatments, but biologics, as the most effective therapy, are thought to be most susceptible to this type of mechanism of disease modification.
The other postulation is that the epigenetic mechanism, in the form of DNA methylation in lesional skin, is normalised in new-onset psoriasis compared to chronic plaque forms.
In conclusion, more specific patient profiling is required in order to find the most successful treatment option, or rather to have the maximum effect on modifying the course of the disease.
Speaker: Dr. Hok Bing Thio (Rotterdam)
In the next presentation, Dr. Hok Bing Thio looked at population differences in psoriatic patients. In his presentation, he stated that there are no differences in prevalence between men and women, but that there are differences in age of onset, with a higher prevalence in adults compared to children.
Although the general population prevalence is around 2%, Dr. Bing Thio addressed the fact that there is a higher prevalence in more economically developed countries (high income countries), with examples of higher percentages of psoriasis patients in Australia, Europe and North America. Lower prevalence was found in East Asia, with the lowest prevalence in Taiwan.
In contrast to a lower prevalence rate, the clinical forms of psoriasis in these countries are more severe, with higher PASI scores and BSA affected. Also, the clinical forms in dark-skinned and Asian patients are different, with the colour being more violent than the erythematous, which is the clinical hallmark of fair-skinned patients.
This difference in appearance and severity of the disease can be explained by the variance in the expression of human leukocyte antigen alleles in certain ethnic groups. For example, in cases of HLA Cw6, expression is most common in the general population. However, in cases of HLA Cw1, which is more common in the Asian population, there is a higher incidence of pustular and erythrodermic psoriasis. With these differences in gene expression, there is a variable response to systemic treatment that should also be considered. In addition to gene expression, the triggers of psoriasis can be influenced by the geographical location (environment) of a particular ethnic group, but also by their lifestyle (type of diet, lifestyle, stress).
Differences in diet make the microbiome variable, so some food preferences can induce dysbiosis and cause mild but chronic inflammation in psoriasis.
Dr. Bing Thio further postulated that there are differences in immunometabolism, more specifically mitochondrial activity, between different populations, which is an important factor in the exacerbation of psoriasis. He then focused on medication that can modulate mitochondrial activity, such as metformin and semaglutide, and gave case examples of psoriasis improving in a patient on ozempic.
In the final slides of his talk, he highlighted the importance of air pollution and its effect on psoriasis by also modifying mitochondrial activity.
In summary, we can conclude that genes, epigenetics, microbiome and mitochondria (plus pollution) are the key factors influencing psoriasis, but with differences in expression between races.
Speaker: Prof. Hervé Bachelez (Paris)
In the introduction, Dr. Bachelez hypothesised that there may be a link between pustular and plaque psoriasis. A clear link was established by the fact that almost half of patients with generalised pustular psoriasis had previously had chronic plaque psoriasis. The same association was found for patients with palmoplantar psoriasis, psoriasis vulgaris and psoriatic arthritis.
More specifically, the first gene expression association was made with CARD14 gain-of-function mutations, which were "hot spots" in more than 60% of patients with psoriasis vulgaris, pityriasis rubra pilaris and generalised pustular psoriasis. In cases with CARD 14 mutations, spontaneous psoriasis-like skin eruptions occur due to an increased keratinocyte response to IL17A. Patients with these types of mutations may also benefit from biologic treatments with secukinumab and ustekinumab.
IL36 loss-of-function mutations are mainly found in generalised pustular psoriasis, but not in plaque psoriasis. Therefore, patients with generalised pustular psoriasis who have IL36 mutations do not have a history of chronic plaque psoriasis.
Although Dr. Bachelez's first hypothesis was that pustular psoriasis is either driven by an IL17A pathway or an IL36-only pathway, he went on to say that this was completely wrong. There are twenty different immunological pathways that are the same in both pustular and plaque psoriasis, but the degree of upregulation differs significantly between the two. In his presentation, he points to the interferon-I-driven response, which is expressed in a high percentage, more so in palmoplantar pustulosis, but also in psoriasis vulgaris. With this dominant response, patients are more resistant to any type of biologic therapy and small molecule therapy, with the exception of JAK1/TYC2 inhibitors.
This approach can be confirmed by the studies carried out on patients receiving spesolimab, where less than 50% of patients had complete clearance of pustules, concluding that pustular psoriasis is not a disease driven solely by IL36.
Finally, he concluded that we need more knowledge of gene expression and the causes of inflammation, raising the question of whether there should be a change in taxonomy (different endotype of pustular lesions), paving the way for more precision medicine approaches.
Speaker: Dr. Satveer Mahil (London)
The final presentation of the session was given by Dr. Satveer Mahil, who explained how we can personalise treatment for each patient, with data-driven information leading to prevention, targeted treatment and improved long-term prognosis.
The concept of an individualised approach is to explore the potential of biomarkers in each patient that can give us a predictive role in assessing the risk of serious disease, thus giving the opportunity for early introduction of targeted treatment and therefore ideal therapy.
The later part of the presentation focused on the durability of modern therapy, more specific biologics, based on real-world data. The effects of biological treatment should be based on certain biomarkers, but none have been identified as sufficient to be used in daily clinical practice. One of the factors that could predict treatment response was serum drug levels. In the case of adalimumab, drug levels at 4 weeks can predict response at 6 months. With these findings, we can predict a required adalimumab concentration at 4 weeks of treatment necessary to achieve a PASI 75 response (therapeutic range 3.2-7 ug/L), making a reach into clinical guidelines and real-world practice.
In addition to biomarkers, polygenic risk scores may also influence disease severity, the latter being influenced by early age of onset, obesity, smoking and alcohol consumption.
The presentation included data showing that early intervention with guselkumab improves outcomes when comparing short (<2 years) to long (>2 years) duration of psoriasis. The results also show that early intervention can lead to dose reductions while maintaining the results achieved to date. All of this points to the upcoming study to be conducted in the UK with the aim of reducing the long-term drug burden on patients. The trial has the potential to individualise dosing for each patient.
Speakers: Assoc. Prof. Marieke Seyger, Prof. Amy Paller, Dr. Joan Garcías Ladaria, Dr. Peter Hoeger.
Report written by Dr Jovan Lalosevic, M.D., Ph.D.
Speaker: Assoc. Prof. Marieke Seyger (Nijmegen)
The lecture included a short introduction to the possible differential diagnosis of paediatric psoriasis and Prof. Seyger gave us some hints about possible skin signs that can give us a clue to make a better diagnosis of psoriasis. She included the 7 predictive sign criteria (Burden-The et al. DIPSOC study, Br J Dermatol 2022), consisting of:
The next presentation was on updates in the topical treatment of paediatric psoriasis, which reviewed the previously used topical steroids, calcineurin inhibitors and calciportiol, but also introduced new treatment options in the form of 0.3% roflumilast cream (PDE-4 inhibitor) and 1% tapinarof (AhR modulator), which showed promising results (Lie et al, Pediatric Drugs 2024). Prof Seyger also emphasised that ditranol/antralin can have a significant effect on paediatric psoriasis, but that adherence is the main problem with this treatment option (Aoki et al, Dermatol Clinics 2024).
The opinions of patients, parents and physicians may differ. The non-physicians want a quick and safe treatment with complete resolution of the skin lesion, whether the medical staff is less strict about the criteria regarding the effectiveness of the prescribed treatment.
The available systemic drugs for paediatric psoriasis include retinoids, cyclosporine A and methotrexate (Bruins et al, Acta Derm Venereol, 2022), which can give up to 50% of treated patients a PASI 75 response. Apremilkast is a new drug available for the treatment of paediatric psoriasis, with similar results to conventional systemic therapy (Fiorello et al, J Am Acad Dermatol, 2024).
All of the available biologic drugs that are available for paediatric psoriasis are used from 6 years of age, with the exception of etanercept, which can be used from 4 years of age.
The biologics give us a much more effective response, with the majority of patients achieving PASI 75,90 and even around a third achieving PASI100 (Bodemer et al. JEADV 2020). They are also more effective and safer than methotrexate (Sun H et al, Pediatric Dermatol, 2022).
The drugs currently in phase III trials for paediatric psoriasis are cetrolizumab, guzelkumab, risankizumab, tildrakizumab, bimekizumab and an oral deucravacitinib (www.clinicaltrials.gov).
Finally, the professor shared her data on the factors present in paediatric patients who have a greater risk of a severe psoriasis and therefore have a potential need for systemic treatment (in her opinion start first with methotrexate and then with biologics) that are male sex, nail involvement and obesity.
Speaker: Prof. Amy Paller, MD, MS (Chicago)
We know that topical steroids are the cornerstone of topical treatment in atopic dermatitis. The thing that is a variable in the management of patients with mild to moderate AD is the maintenance treatment, either going to a less potent topical steroid, applying a proactive 2-3x per week treatment with either topical steroids or topical calcineurin inhibitors. More recently, topical PD4 inhibitors give us an option for safe and effective treatment of more sensitive areas with 2% crisabolol as the first developed and subsequently 0.15% roflumilast as the FDA approved topical treatment for AD. Newer topical treatments include 1.5% ruxolitinib (JAK inhibitor), which has shown efficacy in moderate AD (Boguniewicz et al, Ann Allergy Asthma Immunol 2018).
Topical non-steroidal anti-inflammatory drugs are becoming more available, and topical calcineurin inhibitors are now approaching their 20th anniversary, with the previously thought risk of lymphoma development now known to be infinitesimally small.
Prof. Paller went on to discuss the efficacy of 0.15% roflumilast in patients older than 6 years, pointing out that even though it is a cream it does not cause irritation as was the case with crisaborole (Eichenfield et al, presented at ACAAI 2023).
Tapinarof 1% cream (aryl hydrocarbon receptor agonist) has shown efficacy in two phase 3 trials (ADORING 1 and 2), with mild side effects, mostly in the spectrum of folliculitis.
For patients who require systemic treatment, the first-line therapy is dupilumab in countries where it is available (Butata, Paller, Ann Allergy Asthma Immunol 2022). In countries where it is not available, patients should be treated with methotrexate, cyclosporine A, azathioprine, mycophenolate mofetil, with the addition of narrow-band UVB therapy if it can be combined.
New and anticipated biologic treatments include IL13 inhibitors (tralokinumab, lebricizumab), similar to dupilumab but potentially with a lower incidence of conjunctivitis; IL31R inhibitors (nemolizumab), focusing on the itch segment of the disease (has to be combined with topical steroids); OX40L inhibitor (amlitelimab), affecting antigen-presenting cells.
Further to her presentation, Prof. Paller stated that she usually switches from one systemic drug to another within 2 months of treatment, and when considering when and if to extend the treatment interval or lower the dose, she pointed out that the patient needs to be "doing well" for at least a year. There was also a comment about the pain of injections given, mostly due to the volume of the drug, where education about appropriate administration is key to reducing unwanted pain.
Avoiding injections opens the door for systemic JAK inhibitors, upadacitinib, baricitinib, abrocitinib. They are as effective as the biologics, have a rapid and strong effect on pruritus and are good at managing flares. Their main issue is the safety profile, with frequent laboratory requirements, neutropenia and hypercoagulability scenarios. In general, they have a broader immunosuppression compared to the biologics, so they are not the first choice for systemic treatment.
Speaker: Dr. Joan Garcías Ladaria (Mallorca)
The definition of hidradenitis suppurativa (HS) is the presence of "typical lesions" (papules, nodules, draining sinuses) affecting specific regions (flexural sites) with 2 or more flares in a 6-month period. There are two peaks of incidence, in children around puberty (10-12 years) and in adults around the age of 25, with a second peak around the age of 40.
The main problem is that hidradenitis suppurativa is an under-diagnosed disease in many countries of the world, and most paediatric patients are not diagnosed until adulthood. The approximate delay in diagnosis in paediatric patients is about 2 years, and a delay of more than 1 year is associated with a more severe/disseminated disease (Liu-Wong et al, JAMA Dermatol 2021). Follicular occlusion and follicular damage are central to the pathogenesis of the disease.
The most common comorbidity in paediatric HS is Down's syndrome (2.7-5% of cases), with a female predominance and a strong association with insulin resistance, obesity, smoking and hormonal imbalances (precocious puberty and PCOs).
Unlike other chronic inflammatory diseases, early age of onset does not correlate with more severe disease (Krueger et al, Br J Dermatol 2024).
There are no universally accepted treatment protocols for paediatric HS, so clinicians use treatment protocols developed for adults. The aim of treatment is to relieve symptoms and prevent disease progression. However, we recommend general measures such as reducing friction, avoiding close shaving and reducing body weight if necessary. Treatment is based on a combination of medical and surgical interventions.
One scale used to assess the severity and management of the disease is the Hurley scale (mainly a surgical scale), other scales (IHS4) take into account different clinical aspects of the lesion (follicular or inflammatory) (Zouboulis et al. Dtsch Dermatol Ges, 2024, Martorelli et al. J Eur Acad Dermatol 2020).
Treatment of paediatric HS should be based on the phenotype of the lesions, whether they are follicular or inflammatory (Melgosa-Ramos et al Actas Dermosifiliogr. 2024). Patients with a mixed phenotype are better candidates for biologic treatment to achieve disease remission.
Special considerations in the treatment of paediatric HS should include the use of tetracyclines (after the age of 8), finasteride in girls before menarche, isotretinoin in cases of concomitant acne, and secukinumab can be tried as an off-label use in children older than 6 years.
Finally, Dr Garcia Ladaria concluded that paediatric HS mirrors adult HS and requires a holistic approach, treating inflammation, identifying comorbidities and avoiding exacerbating factors.
Speaker: Dr. Peter Hoeger (Hamburg)
Newborn babies have the same number of sebaceous glands as adults, but they are distributed over a smaller area. They are also more visible in the first month of life due to maternal androgen stimulation, colloquially known as mini-puberty.
The spectrum of acneiform disorders in prepubertal children consists of
Prepubertal children may have rosacea-like conditions, the most common of which is perioral dermatitis, which in the majority of cases is caused by topical steroids or the use of fluoride toothpastes.
Another rosacea-like condition is idiopathic aseptic facial granuloma, which is often associated with other rosacea-like conditions. The condition has a high rate of spontaneous resolution. It can be treated with anti-inflammatory oral antibiotics. Full-blown rosacea with ophthalmic manifestations is rare and should be treated as in adult patients.
Moderation: Prof. Diamant Thaçi.
Speakers : Prof. Sonja Ständer, Dr. Sarina Elmariah and Dr. Andrew Pink.
Report written by Dr Jovan Lalosevic, M.D., Ph.D.
The main goal of any therapy is to relieve the patient from pain and to reduce itch, whether it is prurigo nodularis or atopic dermatitis.
Among the tools that can help us in this task are the unidimensional itch intensity scales, the numerical rating scale being one of the most accurate (Stander et al, J Dtsch Dermatol Ges, 2022).
What is considered a meaningful reduction in NRS itch:
One of the main expectations of every patient is the fast reduction of itch, and more than 50% of all patients with prurigo nodularis expect it to be in the first month of treatment introduction.
When we look at the expert’s consensus on the management of prurigo nodularis, we can see that it includes:
as well as systemic neuromodulating drugs such as gabapentin, antidepressants and systemic immunomodulating drugs such as methotrexate, azathioprine, cyclosporine A and, in recent years, systemic JAK inhibitors, dupilumab and a new promising drug, an IL-31 signalling inhibitor.
Until now, most patients with prurigo nodularis have not been satisfied with the prescribed treatment. New therapies that are proving effective in reducing itching and nodules are dupilumab (IL4/IL13) and nemolizumab (IL31a) (FDA approved).
Atopic dermatitis (AD) is a chronic and multifactorial disease in which pruritus is a key element, but in most of the moderate to severe forms it is often accompanied by pain. In these cases, the narrative is always directed towards systemic immunomodulatory treatment.
The new treatment armamentarium includes either biologics or systemic JAK inhibitors. Dupilumab is one of the first widely used and effective biologics in AD, with recent studies showing efficacy of tralokinumab, lebrikizumab and nemolizumab in combination with topical steroids and/or topical calcineurin inhibitors. At the other end of the spectrum, the development of JAK inhibitors is giving us even greater effects on pruritus, with abrocitinib, upadicitinib and baricitinib showing efficacy in the 4-point reduction of itch on the NRS.
In the only head-to-head study comparing upadacitinib with dupilumab (Blauvelt et al, JAMA Dermatol, 2021) in adults with moderate to severe atopic dermatitis, upadacitinib showed superior and faster skin clearance and itch relief with tolerable safety.
Chairs: Dr. Vincenzo Bettoli, Dr. Lajos Kemeny.
Speakers: Dr. Nicolas Kluger, Dr. Layos Kemeny, Dr. Vincenzo Bettoli, Dr. Margarita Larralde.
Report written by Dr Jovan Lalosevic, M.D., Ph.D.
Speaker: Dr. Nicolas Kluger (Helsinki)
Transgender people undergoing masculinising hormone therapy experience a wide range of dermatological effects during the initiation and maintenance of testosterone therapy. The role of hormone therapy is to reverse or reduce the physical sexual characteristics of the sex assigned at birth and to enhance and build up the characteristics of the expressed sex, and these therapies apply to both transgender and gender non-conforming patients. Acne is one of the most common side effects for many transmasculine patients receiving testosterone. Acne can worsen body image and mental health and has a significant impact on the quality of life of transgender patients.
Discussing the condition with patients should be neutral, using terms that may not trigger gender dysmorphia (chest, genitals).
Treatment should be the same as for cisgender patients, taking into account the risk of pregnancy in female transgender patients.
Speaker: Dr. Layos Kemeny (Szeged)
Is there an unmet need for new therapies? was the first question asked by Prof. Kemeny asked in his presentation. Even though we have very effective forms of treatment, patients are asking for treatment that is fast-acting and has a long-term effect without relapse or scarring.
New therapies could include targets such as inflammatory cytokines.
Cutibacterium acnes strains can induce different th17 responses in the skin (Agak et al, JID 2018). All this leads to the question of which cytokines can actually be targeted, such as IL1beta, IL17A and TNF alpha.
Clinical trials have not shown efficacy in blocking inflammatory cytokines with antibodies in the case of IL1beta and IL17. However, the efficacy of anti-TNF, IL-13/23 and IL-23 has been demonstrated in case reports of acne and acne-associated syndromes.
The question that arises is whether these cases are really acne, but perhaps a facial type of hidradenitis suppurativa (conglobate type).
The other way we can influence the pathogenesis of acne is by modulating C. acnes towards the non-acne causing strains, either by neutralising antibodies against CAMP or by overpowering it with other bacteria (Karoglan et al, Act Derm venreol 2019, Labeer et al, Cell Resp Medicine 2022). The latest effort has been demonstrated with topical phage therapy in a mouse model (Rimon et al, Nat Commun 2023).
Ultimately, no biologics are approved for the treatment of acne. For treatment-resistant acne, think of HS. For severe therapy-resistant acne, with or without acne syndromes, you could try blocking TNF-alpha, IL-17 or IL-23. Modulating the skin microbiome may be a new way to improve acne.
Speaker: Dr. Vincenzo Bettoli (Ferrara)
The definition of a low dose may vary. A common definition is that a low dose is one that is lower than the standard dose (in the case of isotretinoin, this is 0.5-1 mg/kg). A low dose can also be thought of as being lower than the highest dose that a particular patient can tolerate (the dose that a patient can tolerate without side effects).
Low-dose isotretinoin can be given continuously or intermittently (alternate days, alternate weeks). Treatment can either start with a low dose followed by a gradual increase to a maximum tolerated dose, or it can start with a high dose and then be reduced to a low dose due to poor tolerance of side effects.
Each individual patient has a different metabolism and bioavailability for drugs, therefore patients will develop side effects (cheilitis being the most common) according to their personal dose dependency.
Patients on treatment can develop flares, which are often related to a dose that is too high for them. Studies show that starting with a low dose and increasing to the highest tolerated dose significantly reduces the frequency of severe flares (Bettoli et al. Dermatology, 2009). The dose should be increased each week, up to a daily increase of 10 mg. This dosing regimen has demonstrated efficacy while minimising treatment side effects.
Notwithstanding the lower incidence of side effects, low-dose isotretinoin is better tolerated, equally effective in the long term, and procedures such as peels or lasers are easier to perform while on it.
Low-dose oral isotretinoin has its drawbacks, with women needing to use contraception for longer and taking longer to clear their acne.
Relapses are more common with low-dose isotretinoin, but some patients experience them more frequently, and these are
Finally, Dr Bettoli gave us his treatment preference:
Speaker: Dr. Margarita Larralde (Buenos Aires)
Light treatment for acne can be divided into treatment of active lesions (IPL and PDT) and treatment of acne sequelae (CO2, Q-switched, IPL). The mechanisms of action of light-based therapies may include
Intense pulsed light improves inflammatory acne and reduces the size and number of glands by direct phototoxic damage, reducing their density and sebum production. It may also exert an anti-inflammatory effect by down-regulating tumour necrosis factor and up-regulating transforming growth factor beta1 signalling. IPL also corrects vascular dilation, resulting in a reduction in erythema in inflammatory acne.
A new light device that has been used for mild to severe inflammatory lesions is a diode laser wavelength of 1726nm that performs selective photothermolysis of the sebaceous glands. It is combined with prior, parallel (during energy delivery) and post-cooling of the superficial epidermal dermal structures to ensure safety and minimal patient discomfort.
Another device that can be used in the treatment combines vacuum and broadband light technology. Vacuum deep cleanses the pore by extracting the blocked sebaceous material. Broadband light targets porphyrins, destroying the C. acne bacteria, reducing sebum production and reducing the erythema and pigmentary changes associated with acne.
The fractional Q-switched 1064 nm laser is often used to treat acne scars.
Conclusion:
Speaker: MD Karina Polak (Katowice, Poland) Kozik A et al. Skin lesions in runners – systematic review. e-poster 1883
In this Olympic year, it was expected to read about some sports during this EADV congress.
I was very pleased to find that the polish team from Katowice performed an exhaustive review of the skin associated issues that can affect runners. An enormous range of dermatological changes can affect runners.
They mainly include:
Prevention strategies include properly educating runners, using moisture-wicking fabrics, applying anti-chafing products, maintaining good hygiene, wearing sunscreen, and staying mindful of environmental conditions to mitigate these risks.
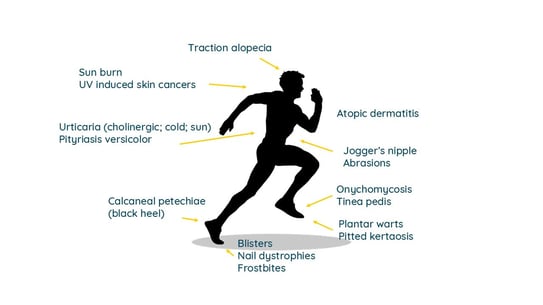
Figure 1. Skin associated issues in runners
Speaker: Dr. Bruno Halioua (Paris, France)
Skayem C et al. Use of magnetizers and traditional healers by people with skin diseases: A worldwide study ALL Project. e-poster 3447
Musa BS et al. The use of non-conventional medicines among adult dermatology patients attending a tertiary facility in Northern Tanzania. e-poster 2740
Fikri C et al. Acute Localized Exanthematous Pustulosis induced by topical herbal medicine. e-poster 0243
El Fekih I et al. DRESS syndrome in a Chinese induced by Chinese herbal medicine. e-poster 0290
It is known that some patients may decide to turn to “alternative” or “complementary medicine” to treat their chronic conditions.
Healers and magnetizers are among those alternative healers that allegedly influence a person’s energy fields to enhance their health. A worldwide survey, including 20 countries and a representative sample of the general population of each country, enrolled 50,552 individuals, among which 35% (n=17,627) had one skin disease or more. Among these respondents, for the purpose of this study, the researchers analysed a population of 12,485 individuals. The main skin conditions were acne (35.6%), atopic dermatitis (AD, 20.1%), psoriasis (7.9%), rosacea (3.9%), vitiligo (1.4%) and hidradenitis suppurativa (HS, 0.8%). The researchers found that 3.1% of the respondents reported consulting magnetizers or traditional healers to treat their conditions. The use of magnetizers/healers was more frequent among the young and urban residents. The highest prevalence was in India (8%), UAE (5.2%), South Africa (4.9%); China (4%), Kenya (3.6%), South Korea (3.5%) and… France (3.4%). The prevalence in Europe was 2%. Patients with vitiligo and HS disease were most likely to go to magnetizers/healers. Thankfully, in most cases the use of a healer did not interfere with the medical management (66.3%).
It is notable that patients with vitiligo and HS are those who most turn toward those alternative healers. The explanation relies most likely in the lack of fully efficient therapies thus far in notoriously resistant diseases. With therapeutic progress, the patients might not go to those healers.
A second poster addressed similar issues in Northern Tanzania. A monocentric study showed that 35.5% of the respondents (out of 414) had consulted an alternative medicine provider for acne (64.7%), psoriasis (63.6%), AD (52.3%), pigmentary disorders (47.8%) and blistering disorders (43%). Alternative medicine included African traditional medicine and home remedies.
However, alternative medications such as phytotherapy/herbal treatments can be responsible for cutaneous side effects from various severity. This was illustrated by, on one side, a case of self-limited acute localized exanthematous pustulosis due to application of Capparis spinosa to treat sciatalgia on one leg and hip and, on the other hand, a case of DRESS syndrome that prompted hospitalization of the patient and supportive care with systemic corticosteroids after application and oral intake of Chinese herbal medicine preparation that contained 23 different ingredients!
Jain S et al. Characterizing disparities in dermatology publishing: a bibliometric analysis of authorship trends. e-poster 1888
Authors from low and low-middle income (LMIC) are underrepresented in medical literature. However, they belong to countries that share the highest burden of dermatological diseases. Authors value publishing in high impact journals to build scientific rapport and to showcase scholastic productivity, for academic promotion.
American authors undertook a bibliometric analysis to assess the top 6 dermatology journals based on cited impact factor (IF) and search for publications from 2018 to 2023. They extracted publications with authors from low-, low- and middle and upper middle/high income.
Over the last 6 years, only 12% of publications in the highest IF dermatology journals included ≥1 author from LMIC. Less than 10% of publications had an LMIC author in a first or senior position. The most represented countries were China, Brazil, Turkey and Mexico. Authors from LMIC were less likely to be listed as first or senior author.
There is an over-domination of China, India and Brazil.
Explanations for such discrepancies and underrepresentation of LMIC authors in high impact journals include:
Speaker: Dr. Nicolas Kluger (Helsinki, Finland)
Tattoos are getting increasingly popular. With almost 20% of adults having at least one tattoo, one of the issues that has arisen is the risk associated with moles. Tattooing on a melanocytic lesion can trigger traumatic clinical and histological modifications that will lead to excision and analysis to rule a malignancy. Large tattoos may cloud the proper surveillance of patients with atypical mole syndrome or numerous moles. Dermoscopy may be challenging due to the superposition of melanocytes and tattoo pigments.
Hopefully, the development of melanoma remains rare and is still considered to date as a fortuitous event. In almost 80% of the cases, melanoma developed de novo within the tattoos. Fortunately, not all pigmented lesions within tattoos are melanomas. Cases of seborrheic keratoses, warts and spitz nevus have been described. The main common-sense rule is to avoid getting a tattoo on any pigmented lesions. Besides, as a rule, tattoos should not be done over a preexisting lesion without diagnostic. Of course, surgical scar of melanoma should never be tattooed to allow clinical surveillance. In case of doubt, tattoo session should be postponed, or the customer should choose another area to get the tattoo and referral to the GP or the dermatologist is then warranted. Tattooists’ training is also important. Tattooists should be aware that skin lesions should not been tattooed without any medical evaluation. They should leave spaces if they are tattooing in the vicinity of tattoos and leave about 0.5 to 1 cm around each naevus. Thequestion of the role of tattooists in melanoma screening remains open but has also ethical limitations that need to be addressed.
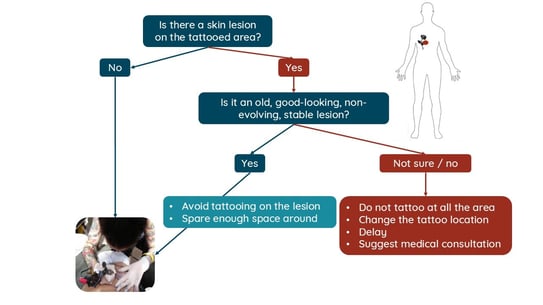

Figure 2. Suggestion of (a) a decisional algorithm and (b) key points for the tattooist in case of lesion on area planned for a tattoo
Loubaris Z et al. Traction alopecia secondary to an oxygen mask in a child: a case report. e-poster 2164
Sharma A, et al. Traction alopecia in the paediatric Sikh population. e-poster 2020
Phiske M et al. Congenital triangular alopecia with eyebrow and lower eyelid alopecia: a hitherto unreported rare association. e-poster 2056
Mansour Billah L et al. Sisaipho: a rare presentation of Alopecia Areata. e-poster 2177
Pappa G et al. Alopecia areata in a pattern distribution: redefining sisaipho under a new pathogenetic perspective. e-poster 2104
Several posters reported surprising clinical presentations of hair disorders.
Loubaris et al. from Rabat, Morocco, reported the case of a 2-year-old girl that had developed areas of alopecia on the occipital and temporal areas. She also disclosed brownish spots on the cheeks and nose matching the mask. Medical history revealed that she has been wearing a high concentration oxygen mask for a week because of broncho-alveolitis. The authors concluded on the role of the oxygen mask in the occurrence of alopecia and diagnosed traction alopecia (TA). No biopsy has been performed and the evolution of the hair condition is unknown.
Sikhism is a monotheistic religion founded in the 15th century in Punjab, India. In Sikhism, uncut hair is considered a symbol of devotion and respect for God's creation. Sikhs keep their hair long, tend to tie them in a tight knot over the vertex and they cover their hair with a turban. Such constant hair style leads to TA in adult Sikhs. In a poster from the UK, the authors reported two paediatric cases of TA in Sikh boys aged 14 years-old with a notable recession of the fronto-temporal hairline and a fringe sign. Parents were advised to avoid putting the hairs in a tight knot for long periods of time, to let them loose as often as possibly open or in a loose bun or ponytail. Local minoxidil 2 or 5% can be tried to stimulate regrowth.
Sisaipho alopecia (“ophiasis” written backward, or ophiasis inversus) is rare clinical variant of alopecia areata (AA), that has been first described in 1996. It presents as a scalp hair loss sparing the temporal and occipital areas, with a possible centrifugal extension. It can be easily mistaken for androgenetic alopecia. So much so that some authors, like Pappa et al. have suggested to rename this clinical presentation “alopecia areata in a male or female pattern distribution” to allow a better recognition of this rare situation.
Involvement of eyebrows and eyelashes is infrequent. Conversely trachyonychia and comorbidities would be more frequent in those patients.
Lastly, Phiske et al. from Mumbai, India reported the case of an 8-year-old girl who presented with bilateral congenital triangular alopecia associated to lower eyelid eyelashes loss and patchy hair loss of both eyebrows.
Jachiet M et al. Dupilumab in Adult Patients with Moderate-to-severe Prurigo Nodularis: 6-months Real-world Follow-up Results from the French Early Access Program. e-poster 3056
Harrison K et al. Dupilumab Is Efficacious in Patients With Prurigo Nodularis Regardless of History of Atopic Comorbidities: Pooled Results From Two Phase 3 Trials (LIBERTY-PN PRIME and PRIME2). e-poster 3076
Prurigo nodularis (PN) is a skin condition characterized by intensely itchy nodules or bumps. It is often caused by chronic scratching due to severe itching, and has a notable impact on quality of life. Dupilumab, an IL-4/IL-13 antagonist, has been approved in the treatment of moderate (> 20 nodules) to severe (> 100 nodules) PN. In France, an early-access authorization allowed prescription of dupilumab in autumn 2022. A poster reported the results of 155 patients that received 600 mg dupilumab subcutaneously (initial injection) and then 300 mg every two weeks. Demographic, disease characteristics, efficacy and safety were evaluated.
Mean age of the cohort was 62.8, including 60% of women. 72% of the patients had comorbidities of any type but only 17% of the patients had atopic comorbidities (asthma, atopic dermatitis, conjunctivitis). Dupilumab has been discontinued only in 11%.
Overall, almost 40% and 73.5% of the patients reached an IGA PN-S score* of 0 to 1 at 3 months and 6 months respectively. Regarding itch intensity, the WI-NRS score (Worst Itch Numeric Rating Scale)** was initially at 7.1 and decreased to 3.5 and 2.8 at 3 months and 6 months respectively and a relative change from the baseline of -46.8% and -50.2% respectively. The quality of life has improved, as shown by the DLQI of 6.5 points and 8.4 points at 3 and 6 months respectively.
The most frequently reported adverse events were headaches and pruritus in 6.8% each.
The improvement in clinical outcomes and the safety profile of dupilumab were consistent with previous studies.
Of note, a pooled analysis of two phase 3 studies that assessed the efficacy of dupilumab (LIBERTY-PN PRIME and PRIME 2) showed that a history of atopic dermatitis had no influence on the outcomes.
*IGA PN-S ranges from 0 (clear, no nodules) to 1 (almost clear, ≤5 nodules), 2 (mild, 6-19 nodules), 3 (moderate, 20-99 nodules), and 4 (severe, ≥100 nodules).
**Worst Itch Numeric Rating Scale (WI-NRS) is a single-item, patient-reported questionnaire designed to measure an individual's “worst itch” (ie, intensity of itch) in the past 24 hours on an 11-point rating scale (with 0 representing “no itch” and 10 “worst itching imaginable”).
Barbarot S et al. Maternal supplementation with prebiotics during pregnancy regulates colonization of the microbiota of high-risk children, but does not prevent atopic dermatitis at one year of age. The PREGRALL multicenter randomized control trial
ETFAD - European Task Force of Atopic Dermatitis
The PREGRALL study is a French prospective randomised trial that evaluated the efficacy of a prebiotic in the primary prevention of the development of atopic dermatitis (AD) in 1-year-old infants.
A prebiotic is an undigested sugar that stimulates the growth or activity of beneficial intestinal bacteria. Prebiotics have direct effects on epithelial and immune cells, but also indirect effects via an increase in Bifidobacteria and Lactobacillus bacteria. Animal studies have shown that administration of prebiotics to pregnant mothers protects offspring from food allergies.
The hypothesis of the PREGRALL study was that taking prebiotics during pregnancy would modulate the foetal immune system and reduce the risk of developing AD in the child. However, it is known that taking prebiotics after birth has no effect on the prevention of AD.
This randomized study included 376 pregnant women at risk of AD in 2 groups: a placebo group (PBO) and a group taking the prebiotic from 20 weeks’ gestation until delivery (188 patients per group). The primary outcome was the prevalence of AD at 1 year of age.
There were no differences between the two groups in either the prevalence at 1 year of age (around 20% in both groups) or the severity of AD. Nor was there any difference according to whether the baby was born vaginally or by Caesarean section, according to breastfeeding mode or according to allergies. However, there was indeed a modification of the maternal microbiota, which was transmitted to the child at the start of life.
It appears that the prebiotic used in this study has no effect on the prevention of AD in the short term. The aim of the study is now to see the prevalence of asthma at 5 years of age.
Prof. Brigitte Dréno from Nantes (France) reported her experience with spironolactone for acne. Spironolactone (SPL) is among the current trendy drugs that can be given for acne patients.
SPL is a synthetic 17-lactone steroid that acts as an aldosterone receptor antagonist, a potassium sparing diuretic and an anti-androgen that targets the sebocyte, inhibits testosterone, dihydrotestosterone, and also 5-alpha reductase, and increase SHBG (sex hormone-binding globulin).
SPL is used at low dose in acne, between 50 and 150 mg daily, in the middle of a fat-containing meal. Patients with inflammatory lesions and previous treatment with isotretinoin respond better to treatment, while those with a contraception with intrinsic androgenic activity of progestin did not respond to the treatment.
Prof. Dréno provided reassuring data regarding safety and tolerance. Side effects of SPL are dose related. SPL is not associated with a risk of hyperkaliemia when prescribed to patients aged 15-45 yo. It is not associated with increased risk of thromboembolic events, breast or uterine tumours, or hypotension.
The best indications for acne patients are those with:
Patients with central hyperandrogenism like with hirsutism and alopecia are not a good indication for SPL.
A recent randomized double-blind trial comparing doxycycline with PBO5% 3 months followed by PBO alone and SPL 150 mg/daily + PBO 6 months showed that SPL
was 1.37 times more effective at 4 months, and significantly more at 6 months than doxycycline. However, doxycycline proved to be more rapidly efficient.
Adverse events were low, including dysmenorrhea.
Overall, there is a body of evidence supporting SPL in acne of women. It is an alternative to isotretinoin. It can also improve pre-pubertal acne, although less than for adults.
It is also important not to stop the treatment abruptly, but to decrease the dose by 25 mg after 6 months.
Speaker: Dr. Aaron Mangold (Scottsdale, United States)
Deucravacitinib in the treatment of lichen planopilaris - interim analysis.
Mahmoudi et al. Efficacy and safety of the oral Janus kinase inhibitor tofacitinib in the treatment of adults with lichen planopilaris: A randomized placebo-controlled trial. e-poster 2064
Lofti et al. Platelet-rich plasma as a new and successful treatment for lichen planopilaris: A controlled blinded randomized clinical trial. e-poster 2066
Lichen planopilaris (LPP) is a lymphocyte-mediated cicatricial alopecia that is difficult to manage and without any effective treatments. The etiology and scarring are poorly understood.
First line treatments include local and intralesional corticosteroids, followed by hydroxychloroquine, or systemic treatments like cyclosporine, mycophenolate mofetil or methotrexate.
In cutaneous lichen planus, type I and type II interferon pathways are thought to be accessible to JAK inhibitors. Besides, Th17 cells may play a role in the process.
Dr Aaron Mangold presented the interim results of a small open-label single-arm study that sought to evaluate the safety and efficacy of Deucravacitinib, a TYK2 inhibitor, in adults over 18 yo with a biopsy-proven active LPP. The dosage was 6 mg twice a day. PGA score, lichen planopilaris activity index (LPPAI) and other secondary measures, such as DLQI, and evaluation of itch or Skindex were analyzed. The endpoint of the study is planned at 24 weeks, but only intermediate results at 12 and 16 weeks were presented.
Ten patients have been included with a mean age of 61.4 years, 70% of women, all white. The median duration for the disease was 6.4 years.
Compared to baseline, there was a clear improvement of the LPPAI, with a drop from 3.8 to 1.6 (60% improvement) and 1.2 (70% improvement) at week 12 and 16 respectively. PGA score had also improved at week 12 and week 16. Deucravacitinib was well tolerated with no drug-related serious treatment-emergent adverse event, no discontinuation. 70% of the patients had acne. Deucravacitinib improved the disease activity and PGA in a small cohort of patients at week 12 and 16. Improvement is seen at 3-4 months. Additional comparative studies are needed to better assess safety and efficacy.
A randomized multicenter double-blind placebo-controlled trial from Teheran, Iran, included 37 patients (26 women; mean age 45 yo) attempted to assess the safety and efficacy of a JAK inhibitor, tofacitinib. However, the study took place during 2020-2022 and COVID-19 has clearly impacted the study, in the placebo group mainly.
Another study from Iran compared highly potent corticosteroid (clobetasol) with platelet-rich plasma (PRP) in combination with clobetasol in 24 participants. Interestingly the PRP group had better outcomes regarding LPPAI and patients’ satisfaction. Both treatments were well tolerated. The mechanisms of action of PRP in LPP are unknown. Work hypotheses include a stimulation of hair follicle stem cells and a reduction of inflammation. However, the results in the literature regarding PRP in LPP have showed mixed results. PRP may be of interest, but more robust data are needed to define its place in the arsenal against LPP.
Speaker: Dr. Martina L Porter (Boston, United States)
Ruxolitinib cream for mild-to-moderate hidradenitis suppurativa: 32-week data from a randomized phase 2 study.
Hidradenitis suppurativa (HS) is a debilitating inflammatory skin disease characterized by painful nodules, drainage and scarring in skin folds. Injectable adalimumab and secukinumab are currently the two biologics approved for the treatment of HS. Dysregulation of Janus kinase (JAK)-dependent signalling pathways is implicated in HS, therefore opening the possibility to explore the efficacy of JAK inhibitors.
Porter et al. reported results of a phase 2 study evaluating ruxolitinib cream 1.5%, a JAK1 and JAK2 inhibitor, twice daily in mild-to-moderate HS.
69 adults with Hurley stage I/II HS (mean age 29 yo, mainly women, without draining tunnels, and a total of abscess and inflammatory nodules of 3 to 10 (AN count, mean 5.4) were equally randomized in 1:1 to 1.5% ruxolitinib cream or vehicle (placebo) for a 16-week continuous twice a day treatment, after which all patients applied ruxolitinib cream twice as needed (AN count ≥1 and/or Pain NRS score ≥1) during a 16-week open label extension (OLE). Efficacy was assessed by:
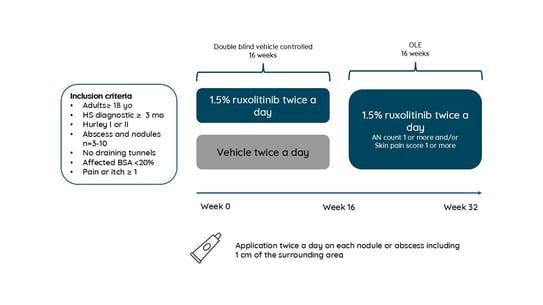
Figure 3. Study protocol
The results are summarized as follows:
the change in baseline of the AN was of almost -3.95 at week 32. The proportion of patients achieving AN50, AN75, AN90 and AN100 was of 79%, 54%, 21% and 21% and 81%, 67%, 19%, and 19% at week 16 and 32 respectively. Over 80% of the patients achieved a HiSCR 50 at week 32. Ruxolitinib was well tolerated.
Application of 1.5% ruxolitinib cream through Week 32 of the OLE study period resulted in sustained or improved clinical signs of HS and was generally well tolerated. Ruxolitinib cream may be a novel approach to address an unmet medical need in the treatment of milder HS.
Speaker: Dr Ayelet Rishpon (Tel Aviv, Israel)
Skin problems in the transgender population
Speaker: Dr Nicolas Kluger (Helsinki, Finland)
Management of acne in transgender individuals
The number of people with gender identity issues seeking professional help increased dramatically in recent decade. In the US 1,3 M adults identify as transgender (Tg, 0,5%) and in the EU, there is about 1,5 M of Tg (Amnesty International, 2015). Transfeminine people (TgF) are people assigned male sex at birth and with a gender identity along the feminine spectrum (includes transgender women and may include non-binary people). Transmasculine people (TgM) are people assigned female sex at birth and with a gender identity along the masculine spectrum (includes transgender men and may include non-binary people as well).
Tg patients may be reluctant to seek medical care as they fear judgmental approaches and stigmatization. Besides, health care providers may not feel comfortable in managing Tg patients because of lack of training and knowledge. In dermatology, Tg patients may avoid skin examination (for moles for instance). Therefore, there is a need for creating an inclusive environment by posting non-discrimination statements, providing gender-neutral restrooms, using gender-neutral language etc.
Acne represents 80% of TgM with skin problems. In 70% of the case, acne is associated with testosterone intake, its overall prevalence is around 26%, with a delay of 12 months after testosterone initiation. Risk factors for acne include younger age at hormonotherapy, BMI, testosterone levels, smoking.
TgM develop hormonal acne in similar locations to other forms of androgen-dependent acne, on the lower third of the face, chest, upper arms, and back. Acne fulminans is rare and may occur with testosterone increase. Binder’s acne is a specific acne of the trunk due to the use of compressive garment to flatten the chest in TgM.
Management of acne in TgM is very close to cis-gender patients according to severity. The specificities include:
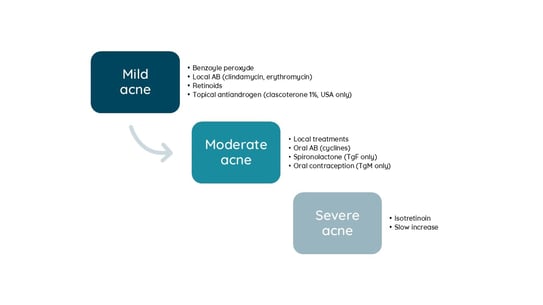
Figure 4. Therapeutic scale for treating acne in transgender individuals
The second dermatological complication is pattern hair loss (avoid using “male” or “female” pattern hair loss). Pattern hair loss is more common in TgM due to testosterone, with delay of onset after testosterone. It can be desired or not. Oral or topical minoxidil can be given for both Tg, 2.5 mg in TgM, 1,25 in TgF. Finasteride and dutasteride can be used as they don’t lower the level of serum testosterone. Some recommend waiting 2-5 years to allow for the development of secondary sexual characteristics. Consider gynecomastia, decreased libido and depression in TgM, but this is debatable. Spironolactone works well in TgF. It is contraindicated in TgM. Other treatments may include PRP, hair transplantation and hairline advancement for TgF.
Lastly, facial and body hair in TgF can be treated by laser hair removal and electrolysis. In TgM topical minoxidil may be used to improve facial hair growth.
Additional roles of dermatologist in gender transition include:
Speaker: Dr. Thibault Mahevas (Paris, France)
VEXAS syndrome (Vacuoles, Enzyme E1, X-linked, Autoinflammatory, Somatic mutation) is a severe autoinflammatory disease recently discovered in 2020. VEXAS syndrome is secondary to the acquisition of a somatic mutation in the ubiquitin-activating enzyme 1 (UBA1) gene in the myeloid lineage. There are 3 main loss of function mutations of UBA1 (c.121A> G; c.122T>C; c121A>C) that will lead to the accumulation of proteins triggering cellular stress and activation of immune pathways. Since its first description in 2020, about 300 cases have been described.
VEXAS occurs in men in 95% of the cases (as the disease is X-linked), with a mean age of 68 years, and combines systemic inflammation, haematological manifestations and inflammation of target organs, of which skin involvement appears to be the most frequent (85%) and often the first.
Haematological involvement included: cytopenia, macrocytosis (95%), myelodysplasia (25-55%) and monoclonal gammopathy.
Inflammation: Fever (65%), weight loss (55%) and elevated CRP (97%)
Other manifestations include relapsing chondritis, eye inflammation, lung inflammation, arthritis and increased risk of thromboembolism.
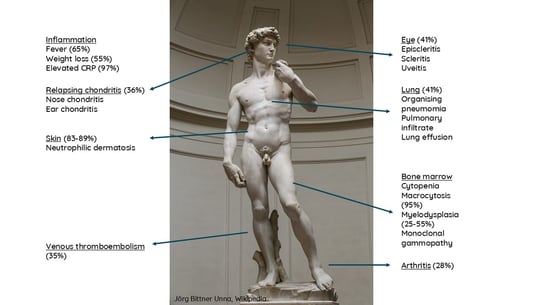
Figure 5. Manifestations of VEXAS
Interestingly, 20 years prior to its individualization, Camille Frances and Jean-Charles Piette from La Pitié-Salpetrière in Paris had reviewed the dermatological manifestations of 200 patients with relapsing chondritis and found that cutaneous manifestations during relapsing chondritis in male was associated significantly with a risk of myelodysplasia. It is highly possible that that subset of patients presented a VEXAS syndrome.
The cutaneous manifestations of VEXAS syndrome are heterogeneous, but frequent (83-89%) and can also be the first manifestation of the syndrome. The diagnosis of VEXAS should be considered in the presence of skin eruption consisting of multiple (> 10) pink or red inflammatory maculopapules and nodules, more rarely pustules, located on the trunk and limbs, sometimes on the face, particularly if associated with arciform lesions observed in a third of cases, in patients aged over 50 with signs of systemic or organ inflammation, or haematological abnormalities. Other symptoms include livedo, pathergy phenomenon, pseudo-cellulitis, and peri orbital oedema. Clinical phenotype and histological infiltrate vary according to genotype and aminoacidic variants.
Histological analysis of VEXAS cutaneous lesions reveals a typical neutrophilic dermatosis infiltrate, but from the histiocytoid Sweet syndrome type rich in immature myeloid cells, often associated with leukocytoclasia with or without vasculitis.
The concept of Myelodysplasia cutis illustrates that non-blastic cells at varying stage of differentiation can infiltrate the skin while a patient had myelodysplastic syndrome cells in the blood. Those patients have a tendency to corticoresistance and a higher risk of acute myeloid leukaemia.
Very recent studies showed skin of VEXAS and myelodysplasia cutis are both marked by the activation of inflammatory pathways related to cytokine signalling, interferon signalling especially.
The current management of VEXAS syndrome include as first line treatment oral corticosteroids, knowing that there is a corticodependence. In the absence of myelodysplasia, JAK inhibitors (ruxolitinib), tocilizumab and azacitidine are 2nd and 3rd line of treatment. In case of myelodysplasia, bone marrow allograft needs to be considered or Azacitidine.
VEXAS is an haemato-autoinflammatory syndrome related to clonal haematopoiesis and the concept of myelodysplasia cutis. The future of the management may rely in targeting the interferon pathway.
Chairs: Dr. PhD Giovanni Damiani & Prof. Lisa Beck
Speakers: Prof. Lisa Beck, Prof. Dr. Dr. Christoph Schlapbach, Marc Vocanson, Dr. PhD Giovanni Damiani
Report written by Dr Stella Michelaki, M.D., Ph.D.
Speaker: Prof. Lisa Beck (Rochester, United States)
Key points:
Dr Beck begins by outlining cardinal features of AD, including inflammation, itch, barrier and microbial abnormalities, which in this engaging session we will discover more about.
In Dr Beck’s opinion, it is probable that we will have, in the future, more extensive answers and research concerning the following dermatological puzzles:
The Atopic Dermatitis Research Network investigated ~1000 patients, aged 1 to 80, split into Staph-positive and Staph-negative patient groups, based on lab results. The Staph-negative group were negative in their lesional and non-lesional skin; AD was however more severe in culture positive lesions. Also, Staph-positive patients were much more systemically polarized towards type 2 immunity. Patients were also asked if they had a history of Staph infections that required treatment with antibiotics: it was higher in the Staph-positive ones, however the negative culture patient group also reported high numbers in this aspect.
Next, Dr Beck discussed investigations focusing on whether positive Staph cultures determined barrier dysfunction. Using the Aquaflex device (a trans-epidermal water loss – TEWL – measuring machine) in non-AD patients that were Staph-negative in various skin sites, and in AD patients. The Staph-positive group showed more barrier dysfunction in the non-lesional skin.
Another intriguing study using the method of stratum corneum assay (Simpson et al.), using 20 tape strips, and after each of the 5 sequential tape strips, another TEWL measurement is done. Again, highest barrier disruption was shown in the culture-positive patients.
Dr Beck continues to educate on research using molecular methods(Kong et al.), presents that the degree of abundance of S. aureus on the surface of the skin correlates with disease flares. Relative abundance of non-Staph bacteria was plotted against specific Staph species. Healthy controls showed rare S. aureus, whereas AD patients at baseline had varying degrees of S. aureus, but during flares this became a higher percentage in AD patients.
Metagenomic studies (Kong et al.) indicate that, in AD flares, typically one strain is present on the skin surface. These strains were studied on mice epithelium, which was noted to become hyperplastic and presented Th2 and Th17 inflammatory response.
A paper looking into anterior cruciate ligament repair (ACL) investigated factors that could increase risk of infections, and the highest odds ratio was noted in AD patients (Kawata M et al.).
The S. aureus abundance leads to microbiome alterations (when one bacterial species is up, others are affected and may go down, therefore causing microbiome changes).
In recent studies 100% of moderate to severe AD patients showed colonization with S. aureus using molecular methods, whereas clinical labs studies have shown 45 – 95% of AD patients to be colonized with S. aureus. (Simpson EL et al., Byrd AL et al., Bin L et al.)
Also, patients with culturable S. aureus showed more Th2 deviation, more severe disease and greater barrier dysfunction and associated with viral complications such as eczema herpeticum.
Another interesting study followed ~3000 patients assessing disease severity over a 3-month period (Staph culture positivity/ Rajka Langeland Severity) (Simpson et al.). The study grouped Staph-positive non-lesional skin, Staph-positive lesional skin and Staph present in both lesional and non-lesional skin. An increase was shown in culturable Staph in all sites.
It has also been shown that up to 60% of culturable AD patients had positive history of S. aureus with antibiotic treatment.
On its surface, S. aureus expresses adhesion molecules that are meant to bind to wound proteins. AD patients have higher expression of those proteins. Therefore, their skin becomes more hospitable to S. aureus binding. (Paller A et al., Deng L et al.)
S. aureus also produces toxins and proteases that can cause keratinocyte or epithelial cell death, disrupt barrier, and more. Recently, V8 protease has been found to be able to activate nerve endings in the skin (PAR1 mechanism), causing itch.
S. aureus releases molecules that induce a robust innate immune response. S. aureus produces super-antigens, some of which alter type 2 immunity.
Dr Beck then proceeds to differentiate between good and bad bacteria.
S. aureus
S. hominis releases peptides that kill S. aureus without harming other commensal bacteria.
A small Swiss cohort (Meylan et al.) studied S. aureus cultures from axillar swabs at different times for up to 2 years of age. The participants that indeed developed AD showed more S. aureus colonization, especially ones at high risk (infants with >1 relative suffering from AD). Interestingly, 2 months prior diagnosis, the AD patients already showed increased S. aureus colonization. This study therefore suggests that S. aureus comes before onset of disease.
An Irish microbiome study showed that colonization with commensal Staph (coag-negative Staph) may lower the risk of AD (Kennedyet al.). On the contrary to the previous study mentioned above, this study did not see an increase in S. aureus preceding the disease.
Dr Beck continues to discuss if type 2 inflammation of epithelial defects could promote S. aureus colonization? If there is Th2 cytokines (4 & 13 mainly studied), they may counteract the ‘good’ bacterial response (Th17). Th2 inflammation also causes lipid changes, reduced AMPs and lastly, an increased expression of S. aureus adherence molecules.
Epithelial defects have been suggested to drive Staph predominance; such as; alkaline pH, increased sodium content, reduced epithelial innate immune response, itch/scratch cycle.
There is nothing currently to specifically target S. aureus. Systemic antibiotics may kill both pathogenic and beneficial bacteria. Coal tar may reduce Staphylococcus and may increase Cutibacterium adubdance. Topical corticosteroids reduce S. aureus abundance. Narrow-band UVB, Dr Beck explains, has unclear effects on S. aureus abundance. Lastly, bacteriotherapy is a promising new development, while S. hominis trial (read out soon) and Roseomonas mucosa study failed to meet primary endpoint and are no longer moving forward.
Oral probiotics appear to reduce risk of AD, but hae not been shown to improve it once patient present the disease.
Based on a cohort study on bleach baths in a pediatric journal in 2009, they were found to be beneficial in AD. Many publications since then however have shown that bleach baths have no effect on S. aureus. An open label study in adults with mild – severe AD showed that there was a clinical, itch and sleep improvement (Huang JT et al., Stolarczyk A et al.)
Dr Beck then proceeds to discuss biologics, beginning with Dupilumab (monoclonal antibody blocking IL4 & IL13), a pediatric cohort study noted (Paller AS et al.) that the Dupilumab study group had lower risk for non-viral skin infections. A randomized, double blind, 6-week, controlled trial including a Dupilumab versus placebo group was carried out. The Staph abundance (measured by PCR) dropped significantly by 3 days and continued to drop until ~4 weeks, then the study proceeded as an open-label trial where the placebo group dropped significantly and bacteria dropped until 16 weeks. Maximum effect appeared to be reached at 16 weeks.
S. aureus reductions correlate with improvements in AD severity (Simpson E et al.).
Tralokinumab (anti-IL13) reduces S. aureus skin colonization (Beck L et al.). This study uses PCR abundance at 16 weeks.
Dupilumab, but not Ciclosporine, leads to lower S. aureus abundance in AD skin (Hartmann J et al.). TREAT Germany (a systemically treated AD patient registry) investigated AD patients, at 3-month post treatment with Ciclosporin or Dupilumab and studied relative abundance of S. aureus. It was found that reduction was pronounced in the Dupilumab treated group and not the Cyclosporin group. However, the clinical improvement was similar in both groups.
Dr Beck closes her lecture by highlighting the fact that S. aureus is key in AD skin microbiome.
Speaker: Prof. Dr. Dr. Christoph Schlapbach (Bern, Switzerland)
Key points:
Dr Schlapbach starts by discussing the immune mechanisms & biomarkers involved in AD. Barrier defects and microbial dysbiosis lead to innate immunity activation, involving Langerhans cells, dendritic cells and keratinocytes. Cytokines are then released, leading eventually to activation of adaptive immunity in dermis, mainly Th2 cells, that drive the expression of IL13, IL22 and more, which then signal back to keratinocytes. Worsening of barrier defects, hyperproliferation and itch occur.
By blocking cytokines that downstream Th2 cells, AD significantly improves. However, upstreaming of Th2 cells is yet to be fully understood. Trials considering IL33 have failed. TSLP also has not proven to be a driver of it.
Dr Schlapbach briefly discusses psoriasis, reminding us of the Koebner effect: alarmins, IL37 activating pDCs, type1 INF that activate dendritic cells (DC), which then lead to release of IL23, activating Th17, which then leads to production of IL17, which eventually causes the disease. Therefore, blocking IL23 in this case is a key target for the treatment of psoriasis. Dr Schlapbach asks, ‘why is blocking IL23 so effective in psoriasis?’. To explore this, subpopulations of Th17 cells must be considered. Mice and translational studies lead to the understanding that subpopulations are grouped into 1) Conventional (express IL17, IL10) and 2) Pathogenic (express IL17, INFγ, GMCSF), and depend on IL23 for activation. Blocking IL17 and blocking IL23 improves AD. Candida infections are reported much higher with IL17 inhibition. This is due to the fact that blocking IL17 blocks it from both the conventional and the pathogenic T cells, in this way the antibacterial immune response is interrupted. Blocking IL23 selectively blocks the IL17 coming from the pathogenic subpopulation, and allows the IL17 from the conventional subpopulation allowing for protection from infection.
Dr Schlapbach suggests that there are conventional (IL4, IL23) and pathogenic (IL13, IL5, IL22, IL9R, IL17RB, PPARγ) Th2 cells. Allergen specific Th2 cells have this pathogenic phenotype.
Biomarkers in AD are discussed. A review by the International Eczema Council listing biomarkers in skin and blood of AD patients, is presented. IL18 is discussed, as it is a surprising biomarker candidate. IL18, Dr Schlapbach notes, promotes type 1 immune response, production of INFγ from NK and Th1 cells. Studies have shown IL18R gene variants are strongly associated with risk of AD development. A GWAS study on a Manhattan plot is discussed. Filaggrin and gene variants of IL13 are associated with AD.
A study suggesting IL18 as a biomarker for AD is explored. Patients were enrolled from birth, tape strips for immune and skin biomarkers were examined at 2 months, then follow-up continued until 2 years of age with the purpose of recording if participants have developed AD and to what severity. Hazard ratio using biomarkers was estimated. If high levels of IL18 on the skin at 2 months of age, the risk of moderate – severe AD was a threefold increase.
A study by Zhang et al. including AD and psoriasis patients of various severity degrees, analyzed skin biopsies, blood samples and performed single cell RNA seek, T cell clonality analysis and looked for which was the most appropriate T cell group best fitting the AD patients. Th2 cells express high levels of IL22, IL13, GATA3, IL17RB, IL9R, IL18R1.
A study examined T cells from AD and non-AD participants and incubated them with various cytokines for which these cells expressed a receptor for. It was found that only IL9 could upregulate IL18R receptors on these T cells. (Schärli et al.)
Blood samples of AD and non-AD patients were examined (Schärli et al.). IL18 levels in peripheral blood were evaluated, AD patients expressed higher levels. Incubating non-AD participant blood with IL9, we can upregulate the IL18R.
Dr Schlapbach notes that IL9 and IL18 induce IL13 secretion from pTh2 cells in AD.
Another intriguing study, (Schärli et al., in revision) Lesional biopsies from active AD sites were studied. The biopsies were categorized into 4 parts; 2 parts were incubated (48h) with IL18 binding protein and 2 control groups, and then cytokine production was evaluated (Multiplex assay for proteins & Cytokine secretion assay). Blocking IL18 signaling showed ability of downregulate IL13 and IL22 production in the supernanats.
A study using tape strips (lesional and non-lesional) in patients presenting with AD flares as well as biopsies showed correlation the epidermal protein level of IL18 with the dermal RNA level for cytokines. High levels of IL18 in epidermis elude to high levels of IL13RNA in the dermis, which is also the case for IL22, but not for IL4. (Schärli et al., in revision)
Spatial transcriptomic studies on lesional and non-lesional skin showed that most of the IL13 expression was noted mostly on the upper dermis, lower epidermis of lesional skin. Cluster analyses showed correlation between cluster IL13 counts and cluster IL18R1 and IL18RAP counts, suggesting a functional link between the two as well.
Speaker: Marc Vocanson
Key points:
Marc Vocanson begins his lecture by highlighting the importance of AD, as 15% of the pediatric population presents it, and it accounts for 1% of all occupational diseases. Skin lesions are induced by the recruitment and activation of allergen specific T-cells. Allergens may be chemicals or proteins, and many may be exposed to environmental allergens but few develop disease.
Then sensitization is discussed and represented in a clear schematic, as follows;
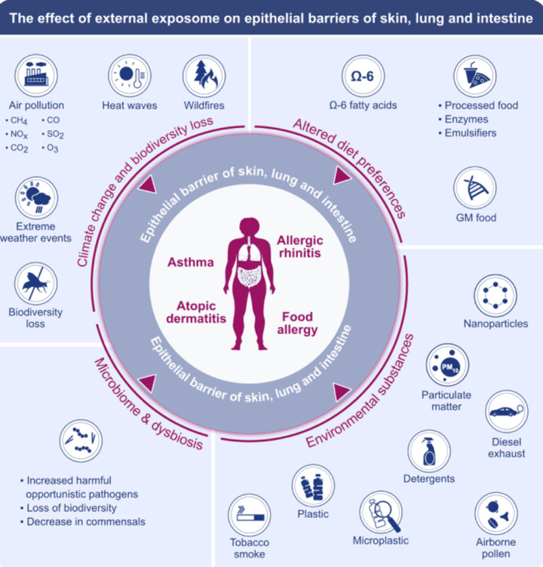
Figure 1. The importance of the effect of the external exposome on the epithelial barriers of the skin, lungs and intestine (Z C Sozener et al., Allergy 2022)
Marc Vocanson distinguishes between primary characteristics of allergens, such as size, lipophilicity, protein reactivity and enzymatic functions. Immune reactions are also influenced by the conditions of exposure, environmental factors, genes, and UV radiation.
Marc Vocanson discusses how UV radiation may impact the sensitization process. A study by Granstein et al., 1983, showed that when mice were exposed to UVB, 3 days before in order to induce the standard allergic reaction to a hapten, in fact the UVB exposure dramatically decreased the induction of an allergic reaction. Collaborations with the team of Prof. Dr. Peter Wolf to study how the skin microbiome modulates the effect of UV radiation on cell response and immunity (Patra et al.). Mice studies have explored the relationship between skin microbiome and UV-induced immunosuppression. What was found was that a lacking microbiome, lead to increased UVB induced immunosuppression and tolerance to DNFB (Vijay Kumar Patra et al.)
Urocanic acid (UCA) is an interesting research topic, as it is typically found on the stratum corneum. In its trans isoform, it turns to trans-UCA and when UV is applied, it is then isomerized into cis-UCA. Marc Vocanson highlights that topical urocanase inhibitors may moderate bacterial metabolism. Moreover, cis-UCA and UVB treatments can also cause bacterial changes. Both trans-UCA and cis-UCA play important roles, including that of natural sunscreens, natural moisturizing factors and help maintain skin pH. Cis-UCA has potent anti-inflammatory and immunosuppressive properties. (PH Hart et al.) Marc Vocanson suggests that both UV-B and cis-UCA exposures lead to transient restructuring of bacterial communities. Interestingly, topical urocanase inhibitors modulate the metabolism of bacteria. Both cis-UCA and UVB treatments showed remodeling in bacteria phyla and species. (Patra et al.) From mice studies, it is suggested that local depletion of skin bacteria increases cis-UCA mediated immune response and therefore leads to enhanced tolerance to DNFB.
Marc Vocanson concludes by mentioning that by eliminating skin bacteria prior to UV light exposure may improve the overall efficacy of phototherapy treatments.
Speaker: Dr. PhD Giovanni Damiani (Milan, Italy)
Key points:
Dr Damiani mentions the existing criteria for AD; Hanifin and Rajka criteria, UK working and Millennium criteria, and when considering them a heterogeneity seem to be reflected on the theory of the Atopic March.
“Chronic syndrome resulting from dysfunctional interaction of ectodermal and endodermal derived epithelia with the external environment (exposome) characterized mainly by dermatological (eczema), respiratory (asthma, rhinitis), ocular (conjunctivitis) and gastrointestinal (food allergies) manifestations” (Kubo M., et al.)
Even though AD is not lethal, the quality of life is impacted greatly.
Dr Damiani mentions that linear progression of Atopic March is partially rejected, in favor of a more complex interplay between genetics and environment.
50% of the pediatric population do not progress from AD into the respiratory and gastrointestinal symptoms (Maiello N., et al.. Children 2022). Also, the classic Atopic March theory largely overestimates allergic comorbidities, especially the gastrointestinal ones.
Recently, a vast scope of comorbidities has been grouped into the VINDICTATE-P mnemonic (Silveberg NB et al.).
Dr Damiani suggests that, in order to understand comorbidities and association measures, we must be aware of the differences between Relative Risk (RR), Odds Ratio (OR) and Hazard Ratio (HR). Then, he proceeds to specify that the term comorbidity means the presence of simultaneous diseases or medical conditions in a single patient. However, the term does not inform about the link between cause and effect, or gives any information on severity. Co-occurrence does not inform about onset of the entities. Lastly severity is not considered when using the term, hence once must be careful when using the term in clinical practice, as Dr Damiani highlights.
In order to interpret statistically significant comorbidities, we must be aware of the time span across which the concurrence of >2 conditions are assessed and of the sequence of disease, which can have serious implications on the prognosis and treatment.
Comorbidity: when there is additional disease in relation to an index disease in a patient
When looking into comorbidities and etiology, Dr Damiani mentions that it is important to consider whether or not there is any etiological association, direct causation, the associated risk factors, heterogeneity, and lastly if disease is independent to risk factors. (Koskinen M et al.)
Multimorbidity is another useful term, describing the situation when there are multiple diseases (>5) in a single patient
Morbidity burden is defined as the overall impact of diseases in a patient, taking into consideration their severity
Patient complexity is the overall impact of diseases in patient, taking into consideration their severity and other health related attributes. (Valderes JM. et al.)
In this lecture, comorbidities are presented as a potential way of presenting complexity. Also, comorbidities and lifestyle have strong correlations. (Solomon I et al.)
The terms secondary (recurrence prevention) and tertiary prevention (to ease impact of current disease) are also explained.
Dr Damiani discusses how disease modifiers may change comorbidities, as an example it is mentioned that Dupilumab can reduce the risk of new allergies appearing by 37% and highlights the importance of new data needed for upcoming drugs. (Geba GBet al.)
Chairs: Prof. Dr. Tilo Biedermann & Prof. Emma Guttman-Yassky
Speakers: Prof. Emma Guttman-Yassky, Prof. Dr. Tilo Biedermann, Dr. Phyllis I. Spuls & Dr. Robert Bissonnette
Report written by Dr Stella Michelaki, M.D., Ph.D.
Key takeawyas:
Speaker: Prof. Emma Guttman-Yassky, MD, PhD (New York, United States)
Prof. Guttman begins her session by saying that AD is moving towards personalized medicine and explains that we need to know the differences between the different phenotypes, especially the new emerging phenotype; adult-onset AD (AOAD).
AD is the most common inflammatory skin condition with lifetime prevalence of 20% and a disease burden that affects daily life in various ways. 5 – 8.1% of adults in developed countries will present with AD.
Prof. Guttman-Yassky notes that AOAD appears for the first time in adulthood. There is a large unmet necessity for therapeutics, specifically targeting AOAD. It is characterized by unique clinical and epidemiological features, compared to pediatric onset AD which persists later into adulthood. The belief used to be that, adults with AD, had it since childhood. However, this is not true, the proportion of adults suffering from AOAD vary from 25 – 50%. Dr Guttman mentions that AD onset is well distributed across older ages.
In 85% of children suffering from AD, disease appears before the age of 5 years. In 20-50% of pediatric AD cases, the disease follows into adulthood, persistence is more likely to occur when the following factors present: later onset, longer disease duration, higher disease severity.
Risk factors for persistence include; long disease duration, later onset, greater severity.
Prof. Guttman-Yassky proceeds to explain that AOAD has a distinct clinical phenotype. Non-typical clinical presentations are more common (prurigo nodularis, nummular eczema, follicular eczema). It has higher association with vesicles and nodules and loss of the outer 3rd of the eyebrows, clinically named as the Hertoghe sign (more common in this patient population). Interestingly, AOAD is associated less with xerosis and pruritus when compared to pediatric AD.
Diffuse eczema is often seen. It may present in in peri-ocular areas. Other presenting sites include the face, neck, scalp, hands and feet.
Studies show that adult-onset AD has greater association with risk factors of smoking & less personal and familial atopic comorbidities than pediatric AD. Other interesting studies shared in the session teach us that pediatric AD and adult-onset AD have common dysregulation of immune genes.
Prof. Guttman-Yassy overviews the main differential diagnoses for adult-onset AD, including:
It is mentioned that in some cases, in order to diagnose, we need to consider biopsy.
Exposures to pollution, diet, stress may differently impact AD course.
AD is a spectrum of endo-phenotypes (immune polarization/ epidermal barrier/ clinical phenotypes: European American AD, Asian AD, African American AD, Pediatric AD, Psoriasis)
It is then highlighted that all AD subtypes share Th2 activation. Different AD phenotypes may need additional cytokine targeting.
Th2 and Th22 define the core AD phenotype in all age groups. (Renert-Yuval et al.)
When atopy initiation appears in infants, IL5 and IL33, IL17 are key players, in adults however, much more Th17 is reported which marks the diseases’ chronicity.
A study aimed to investigate molecular differences in skin and blood, where adults over 20 years old presenting with moderate – severe AD, with onset >20 years, SCORAD of 25 and above, IGA 3 and above, any personal history of AD or atopy was considered to be an exclusion criterium. For the pediatric onset group, criteria were the same and participants had to have onset before 10 years of age. Healthy controls were matched to the patients. RNA sequencing on skin, immunohistochemistry, Olink proseek multiplex assay and RT-PCR for skin markers was performed. The AOAD cohort reported higher rates of Hypertension, Diabetes, Hypercholesterolemia. The pediatric onset group showed greater hyperplasia, increased epidermal thickness, increases in keratin 16 and higher infiltrates in lesional and non-lesional skin. In the global skin transcriptome, high immune activation was noted in both AD groups but the pediatric lesional group showed more inflammation, but the non-lesional was more inflamed in the adult-onset group. Both AD groups shared immune gene dysregulation. The Th2 & Th22 pathways were dysregulated in both AD groups. However, the adult-onset AD showed greater Th1 skewing. In the pediatric AD group greater Th17 skewing was reported. Epidermal barrier defect is much more prominent in the pediatric-onset group. (Facheris, Guttman-Yassy et al.)
Expression of many immune genes correlate with disease severity in both phenotypes.
The serum protemic analysis showed that cardiovascular and atherosclerosis related markers much higher in the adult-onset AD group.
Moreover, blood biomarkers were correlated with SCORAD. In AOAD and POAD, inflammatory and cardiovascular markers positively correlate with disease severity (SCORAD)(PAOD: TSLP, IL4R…).
The Th1 biomarkers correlate in skin and serum of AOAD.
Prof. Guttman-Yassky concludes by noting that AOAD shows higher systemic inflammation and increases in cardiovascular markers with stronger Th1 upregulation in the skin. This is important to consider as some treatments may target only Th2, especially when patients present with atopy on the face.
Key points:
Speaker: Prof. Dr. Tilo Biedermann, MD (Munich, Germany)
Key points:
In recent years, PubMed entries on the topic of AD & microbiome have steeply increased. Mainly, investigations have been focusing on the role of microbiome in the pathogenesis of AD, the role of barrier and immunity and prevention and treatments.
Prof. Biedermann starts his lecture with an overview of the pathogenesis of AD and the importance of normalizing the barrier function and dry skin and type 2 inflammation as well as modulating microbiome in AD. There is indeed an increase in sensitization to food allergies. If filaggrin mutation is present, then the likelihood of peanut allergy is high. When type 2 inflammation happens, the filaggrin is reduced. Vice versa interdependence between immune response and barrier dysfunction. (Brough et al.)
Cytokines, IL4, IL13 can be targeted and may reduce barrier function. (Howell et al.)
Type 17 and type 1 immune responses are disturbed. Especially in the early phase, type 2 cytokines can suppress IL23. However, with overgrowth of S. aureus, there is induction of type 3immunity. Targeted intervention suppresses the type 2 inflammation and leads to restoration of proteins that promote barrier function and responsiveness of antimicrobial peptides.
Dr Biedermann refers back to 2012 and HH Kong et al. research that showed that the basis of AD and microbiome. Treatment of the disease showed to restore the diversity.
A study by Callewaert et al. on Dupilumab and microbiome is discussed. The study showed that, when using this therapeutic, S. aureus dropped significantly and diversity is restored.
Then, an intriguing study on Tralokinumab and S. aureus and barrier function is discussed. Inhibiting IL13 showed this S. aureus reduction and increase in microbiota diversity. (Beck LA et al.)
There is clear interdependence between AD and microbial dysbiosis. (Eyerich S et al.)
Dr Biedermann proceeds to discuss filaggrin mutations. (Clausen ML et al.) Studies showed that if there is mutation, the likelihood of having increased S. aureus on the skin is significantly higher in lesional skin.
Hölge et al. induced the dysbiosis on mice skin in order to identify consequences of microbial dysbiosis on the skin for early AD. Interestingly, IL4 and IL13 producing cells are regulated very early after application of S. aureus.
Lastly, Prof. Biedermann categorizes concepts of intervention into 3 levels: a. barrier restoration, b. inflammation reduction and c. direct microbial modulation.
A study on emollient use in AD & microbiome composition (Glatz M et al.) showed more diversity when emollient is well applied on the skin. Using emollients thus, seem to change the composition of the clustering. However, Dr Biedermann notes that the ‘large’ studies that aimed to show that the use of emollients may prevent AD, have not been successful.
Moreover, the ligands from bacteria can induce immunity but at the same time, some of the ligands also mediate tolerance responses. Could we use this to modulate inflammation?
Studies that explored the idea that the ‘good’ bacteria as a therapeutic strategy (Gueniche et al.) using verum V. filiformis cream versus a placebo group, as a one-month treatment for mild AD, and a highly significant difference between emollient and placebo-treated group.
Non-pathogenic bacteria alleviating AD inflammation induce IL10-producing dendritic cells and regulatory TR1 cells (Voltz et al., J Invest Dermatol 2014). This was another way to show how bacteria may reduce inflammation.
Roseomonas mucosa was isolated and placed in AD skin and reduced AD severity was observed. (Myles IA et al.) Then, in a double-blind placebo-controlled trial, research dove deeper and sadly was terminated eventually, as it failed to accomplish its primary outcome of reducing EASI score by 50%. (Tham et al.)
A study investigating endolysin treatments as a way to fight S. aureus (de Wit J et al.). AD reduction as well as skin inflammation reduction was shown. Then the investigation evolved into a double-blind study which also failed to demonstrate superiority over placebo.
Type 2 inflammation persists and is not reduced, simply by removing the S. aureus overgrowth.
S. hominis strains were studied with the potential of developing antibiotics to directly target S. aureus, especially in combination with antimicrobial peptides. (Nakatsuji et al.)
Future research on the prevention and relapsing of AD is anticipated.
Speaker: Dr. Phyllis I. Spuls, MD, PhD (Amstelveen, Netherlands)
Key points:
Prof. Spuls begins her session discussing the main characteristics of AD, a chronic, non-contagious disease that impacts patients and society. In children the prevalence is up to 20% and in adults up to 10% (GADO 2022).
AD has a spectrum of manifestations, including: erythema, squamae, swelling, red papules, vesicles, exudation, excoriations and lichenification. Therefore, it is important to use validated diagnostic criteria. There is no validated consensus on which diagnostic criteria are to be used in both clinical and research settings.
A systematic review in 2008 revealed already 10 different diagnostic criteria (Brenningmeijer et al.), this systematic review was repeated in 2024 (Muster et al.) and 28 distinct criteria were noted. Prof. Spuls mentions that the most used criteria are the Hanfin and Rajka criteria and has the best sensitivity.
Figure 2. Hanfin and Rajka criteria (Hanifin & Rajka, Acta Derm Venereol 1980)
The UK Working Party Criteria have the best specificity and are the most validated ones:

Figure 3. The UK Working Party Criteria (Williams, BJD 1994)
Prof. Spuls highlights that in order to improve care for AD patients, a consensus on diagnostic criteria is necessary, and they need to be reliable and reproducible in clinical and in research settings.
This is especially important as it is key in order to be able to differentiate from other diseases.
Differential diagnoses include:
It is important to be well educated on the various phenotypes, skin color presentations, distributions and various clinical features.
The phenotypes of AD are listed:
A systematic review on phenotypes is discussed (Bosma et al.), where phenotype was defined as a subgroup of patients with AD. The study included clinical phenotypes only. (Prof. Spuls mentions that there are almost 200 papers only on clinical phenotypes overall). It is vital to reach a consensus.
It is highlighted that it is important to also use core outcome sets in order to evaluate treatment. The work of homeforeczema.org is briefly discussed, and it is an example of a tool which could be used to pool and analyze data from, reducing the waste of research.
The Eczema Area and Severity Index (EASI) is a useful, easy to use, validated tool which however is timely and may not be ideal for phototypes IV-VI.
The EDF Guideline, 2023, starting with baseline treatments, encourages patient education, for instance patients should be educated on triggers of AD such as cosmetics, infection, seasonal influences and stress. Patients should be educated on the etiology of AD (genetics, environment, skin barrier dysfunction, microbiome changes, immune dysfunction). Onto bathing, daily, short baths of 27 – 30oC for the crustae and bacterial removal. Bleach baths may reduce impetiginization. Emollients are to be used in a ‘soak and seal’ method, in order to prevent flares and improve symptoms.
Prof. Spuls, discusses a Cochrane review (Singleton et al.) which summarizes educational interventions and shows that education helps improvement of AD disease course.
Prof. Spuls mentions that at the moment, not all are available in the Netherlands (tacrolimus), so topical steroids remain a necessary tool.
(Silverberg et al.), (Nakagawa et al.)
Interesting studies on topical treatments for AD (such as a Cochrane network meta-analysis and a systematic review & meta-analysis of randomized trials) (S J Lax et al.), included 45.846 participants and 291 studies. However, it is mentioned that it was mainly conducted in high income countries, predominantly in white populations. Only 31 studies were limited to children <12 years old. 68% of studies were industry-funded. Treatment ranged from 7 days to 5 years but the trial participation median was 21– 28 days. Thus, network meta-analysis is only feasible for short-term outcomes which is contradictory when dealing with chronic diseases such as AD. This Cochrane review included; topical corticosteroids, topical calcineurin inhibitors, phosphodiesterase 4 inhibitors, JAK inhibitors, aryl hydrocarbon receptor activators and other topicals, with comparators being other anti-inflammatory agents, and vehicles. Prof. Spuls mentions that the bias was high, mostly due to concerns about selective reporting. The most effective agents were the potent topical corticosteroids, JAK inhibitors and tacrolimus at 0.1%. The least effective were the mild corticosteroids, PDE4 inhibitors and tapinarof 1%. Prof. Spuls notes that information on side effects is missing.
A comparison between the Cochrane review and the Guidelines is helpful, but living data for further analysis would be more helpful.
UV treatment is recommended for adults (NB-UVB, UVA1), but it is largely empiric and there is few evidence-based data.
Prof. Spuls explains that UVB has immune-modulating effects (suppresses cell immunity, activates innate à antimicrobial action), causes thickening of stratum corneum (making the patient less susceptible to pathogens) and lastly, it has an anti-pruritic effect (as it induces apoptosis, inhibits Langerhans cells and can modulate cytokine production).
Starting dose by minimal erythema dose (MED) or Fitzpatrick skin phototype.
Treatments occur 2 – 5 times per week. It is a relatively cheap treatment, thus a good option especially for certain patient groups.
Prof. Spuls asks, how often do we use UV treatment in AD, is it effective and will it be used in the future? A survey (Vermeulen et al.) evaluated 229 dermatologists in 30 European countries and found that 84.7% of clinicians prescribe UV treatment (80.9% as 1st line [NBUVB] and 21.6% as 2nd line [PUVA]). However, it is an experience versus evidence case.
A survey (Steyn et al.) was conducted in 27 European countries, with 114 participants, and found that NB-UVB was mostly available, followed by PUVA and UVA1. In 17% of countries, home NB-UVB treatments were available. However, it is mentioned that there is large variation in prescribing and practice.
A systematic review (Garritsen et al.) showed a preference to NB-UVB and UVA1.
Then a more recent Cochrane review (Musters et al.) categorized patients into short-term phototherapy receivers (<16 weeks) and into long-term receivers (>16 weeks). 32 randomized controls were included, included moderate to severe AD and focused on Fitzpatrick phototype II – IV mostly. NBUVB was mostly reported, followed by BB-UVB, then PUVA, then UVA1. Low certainty of results and risk of bias is mentioned. After 12 weeks, the NBUVB group showed more improvement in clinical signs.
Prof. Spuls outlines the adverse effects, including phototoxic reactions, irritation, burning, bacterial superinfection, eczema worsening and eczema herpeticum.
A submitted study by Knöps et al. on the efficacy and safety of combining phototherapy and topicals in the treatment of atopic eczema, including 29 records on topicals and UV treatment, only 1 report (Tzung et al.) reports on efficacy (tacrolimus with UVB, and combination seems to be better than monotherapy), thus concerns about bias are highlighted, no conclusions on efficacy and safety.
Prof. Spuls proceeds to discuss an ongoing study in the Netherlands investigating NBUVB, including a lot of centers. Also, the BRONTE trial is mentioned (Drucker A et al.) (Canadian AD cohort for translational immunology and imaging, Nested BROadband vd Narrowband photoTherapy for Eczema randomized controlled trial)
Mainly grouped into conventional, biologics, JAK inhibitors and Monoclonal antibody therapeutics.
Prof. Spuls highlights, that physicians must first consider comorbidities, co-medication, child-wish or breastfeeding, infections and more.
On ocular surface disease, it is common, and there is expert consensus on managing Dupilumab-related ocular surface disorders in AD patients. (Achtenet al.)
For conception/ pregnancy/ lactation there is guidance (Vestergaard et al.) and Prof. Spuls suggests updating. Options for this particular patient group include; topicals, UV therapy, Ciclosporin, Prednisone, and there are potential uses for Azathioprine and Dupilumab.
Prof. Spuls, educating on the published and available ‘decision card’ (Vermeulen et al.)
A network meta-analysis on the systemics used for AD (Drucker et al.) includes 98 studies.
Moreover, it is noted that there is need for more studying of conventional treatments. (Flohr et al. investigated the efficacy of ciclosporin vs methotrexate in children and young AD patients, great improvement by both treatments by week 12, methotrexate proved superior in the long-term).
Prof. Spuls mentions that Methotrexate is widely used in AD. A systematic review showed no uniform dosing system, highlighting a potential suboptimal use of the agent. (under-dosing: ineffective, overdosing: adverse effects risk). International consensus dosing regimen for adults and children with AD suggested; starting dose of 15mg/ week, maximum dose of 25mg/week. Lastly, no test dose is needed prior to treatment initiation. (Caron et al.)
Speaker: Dr. Robert Bissonnette, MD, FRCPC (Montreal, Canada)
Key notes:
Dr Bissonnette begins with highlighting the fact that in the US currently, there are some novel AD treatments that are available, whereas in the EU they are not yet. Ruxolitinib and Roflumilast are approved in the US for AD, whereas Tapinarof is approved in the US and Japan for the treatment psoriasis. Also, it is clarified that clinical trials concerning the aforementioned category of drugs have been done in very different settings and patient populations, thus it not scientifically prudent to compare those agents and their clinical trial results. For instance, Ruxolitinib studies were done in adults and Tapinarof ones in children. Ruxolitinib and Roflumilast were focused on mild – moderate AD, whereas in Tapinarof mild cases were excluded.
Ruxolitinib is a JAK inhibitor in cream which is approved in Europe for Vitiligo and in the US for both Vitiligo and AD (at 12 years and older). In the form of oral tablets, it is EMA approved to treat Myelofibrosis, Polycythemia Vera and Graft-VS-Host disease. This is a drug that does penetrate. Statistically significant improvement in regard to pruritus compared to placebo was noted, within 2 weeks the maximum effect was noted. (Papp et al.)
A single centered study treated open label with Ruxolitinib and itch was noted to go down within 15 minutes of application. (Bissonnette et al.)
Roflumilast is a PDE4 inhibitor as tablet. Tablet formulation is approved from the FDA for COPD maintenance in patients with exacerbations. At 0.3% there is a cream formulation approved in the USA & Canada for Psoriasis, but studies for AD were conducted using 0.15%. A recent study (March 2024) a study on Roflumilast in children 2 – 5 years old showed similar results. (Eichenfield et al.)
In regard to itch: from the 1st day, improvement was noted and within about 2 weeks most of the efficacy is seen. (Simpson et al.)
In terms of safety, a slight increase was noted for headaches, nausea, diarrhea and vomiting (in about 1/50 patients). (Simpson et al.)
Tapinarof, a Canadian discovery, is an AhR agonist in cream formulation, which is approved in the US for Psoriasis and in China (as Benvitimod). It increases barrier proteins, ceramide production, decreases cytokine production.
Follicular events (folliculitis with pus, other times may present as keratosis pilaris) and headaches (mostly lasts 1 day) were noted in terms of adverse events. (Silverberg et al., Bissonnette et al.)
In Psoriasis studies, when disease is cleared, patients on average will reappear disease, usually in limited sites, in 115 days. (Strober et al.) Dr Bissonnette thus suggests that when patients clear, clinicians can recommend stopping the drug and revisiting when and if disease returns. Data for AD will be published soon.
Lebrikizumab is an anti-IL13 monoclonal antibody approved for >12 year old AD patients, at 500mg subcutaneously for week 0 and 2. Then the dosage followed is 250mg sc up to week 16. Maintenance dose is 250mg Q 4 weeks. Significant improvement was noted at week 16. Conjunctivitis was noted as an adverse effect. (Silverberg et al.)
Nemolizumab is an anti-IL31R monoclonal antibody, approved in the US for Prurigo Nodularis, and in regards to AD Phase 3 trials have been completed. This trial was done with background TCS. In regards to pruritus improvement was noted within one week and continued to decrease over 16 weeks. In terms of adverse effects, peripheral edema: limbs, bilateral, face edema. (Silverberg et al.)
Dr Bissonnette proceeds to discuss OX40 and OX40 ligand in AD. (Guttman- Yassky et al.)
There is a big interest in developing treatments with this therapeutic. Following T-cell activation, OX40 is expressed, this is important for the survival and proliferation of T-cells.
Telazorlimab is an anti-OX40 monoclonal antibody, that we now know can produce long-term disease control off-drug. Primary end-goal, at highest dose versus placebo, was noted at week 16, in the Phase 2 trial. Did not reach maximum efficacy at week 16, but disease improvement was noted at week 52 and fairly maintained for 12 weeks (Rewerska et al.)
Rocatinlimab is another anti-OX40 monoclonal antibody, it can induce antibody-dependent cell toxicity on T-cells. The maximum dose at Phase 2 was 300mg/2 weeks versus placebo. If the dosing was stopped, efficacy was maintained for about 20 weeks. In terms of adverse effects; pyrexia, chills and aphthous ulcers were reported. (Guttman-Yaskyet al.)
Dr Bissonnette shares new data on Rocatinlimab Phase 3 (Rocket Horizon, study responders at week 24), in terms of EASI75 at time of primary end-goal treated patients reached at 32.8% vs placebo at 13.7% and in terms of vIGA-AD 0/1 treated reported 19.3% response versus 6.6% for the placebo group. Dr Bissonnette notes that he does not believe that maximum efficacy is reached by week 24.
Amlitelimab is an anti-OX40L monoclonal antibody. OX-40L is expressed on antigen presenting cells. The Phase 2 study included various doses for 24 weeks. Primary endpoint for EASI75 started at ~ week 8 and was significant by week 24. When exploring 24 weeks off-drug, maintained IGA 0/1 was 71.9% and for EASI75, 69.0% for those who continued treatment and the participants who stopped treatment for 6 months, showed 57.0% IGA0/1 and EASI75 at 61.6%, therefore, if patient reached IGA 0/1, and then treatment stops, more than half of the patients will continue to present IGA 0/1 after 24 weeks. In terms of adverse effects, no major differences were noted between active and placebo participants. (Weddinger et al.)
Chairs: MD Vincenzo Bettoli & Prof. Dr. Klaus Fritz
Speakers: MD Vincenzo Bettoli, Dr. PhD Øystein Grimstad, Prof. Dr. Klaus Fritz, Prof. Dr. Dr. Klaus
Report written by Dr Stella Michelaki, M.D., Ph.D.
Speaker: MD Vincenzo Bettoli (Ferrara, Italy)
Key points:
Acne scars may emerge from perifollicular abscess rupture and pathological healing. The pathogenesis of scar formation is multifactorial. The content of abscesses is walled off, normally, repair happens within 7-10 days, however further rupture may occur, leading to encapsulation, leading to a deeper abscess and consequently more inflammation which may result in inadequate wound healing, causing scar formation. Fibroblasts are responsible for matrix re-modelling. They release metalloproteinases which degrade the extracellular matrix.
The atrophic appearance of the skin is due to the surface contraction which happens as a result of the reparation of the deeper parts. Moreover, it has been suggested that the overexpression of transforming growth factor beta 1 (TGFβ1) leads to increased degradation of extracellular matrix as well as restricted keratinocyte proliferation, especially in susceptible patients (Berman B et al.). The atrophic appearance of acne scars is due to the irreversible destruction of the sebaceous glands. The activation of IL-2 and of IL-10 have also been linked to the mechanism of scar formation. Plasma cells and B lymphocytes are involved in the formation of infiltrate. Examples of typical acne scars include; bridge, atrophic scars, icepick, rolling and boxcar scars. Super boxcar scars are defined as scars >4 mm in size (Jacob et al.). In case of papular acne scars, there is perifollicular fibrosis and the destruction of elastic fibers.
Dr Bettoli explains that the severity and duration of inflammation are key factors in scar formation. Studies have shown an association between persisting antigen presentation, delayed inflammation and scarring. Less scarring is typically associated with marked T-cell response and intense and shorter inflammation duration. For the prevention of scars, it is important to treat aggressively and catch disease sequalae (scars, erythema, hyperpigmentation) and relapses, as Dr Bettoli remarks.
Onto some statistics, studies have highlighted the impact of scars in acne patients, as it is an aesthetically relevant problem in at least 22% of patients. (Layton AMet al.) Studies mentioned present increased risk of suicide and depression for patients suffering with acne (Rossi AB et al., Cotteril JA et al.). 95% of patients present with acne scars in the face (male:female). In the trunk, scars usually affect men more. Dr Bettoli also mentions that similar acne lesions can produce very different acne scars, depending on the location, typically we see; icepick scars on the face and hypertrophic and perifollicular elastosis seen more on the back.
Dr Bettoli proceeds to discuss risk factors, such as severity, family history of acne scaring and picking behavior, associated with scarring.
Types of scars and their recommended treatments are further discussed. For icepick scars the treatment goal is deep and focal collagen stimulation. Treatments of highest efficacy include; punch excision with suture closure and 100% TCA Cross. Rolling scars have been reported to respond best to Fractional CO2 and Er:YAG or repeated non-ablative resurfacing. Boxcar scars are best treated with punch excision followed by punch elevation. Treatments may be multiple, and patients should be advised that only a certain percentage of improvement may be accomplished. There is limited data on treating papular scars and there is one published study of successful treatment with Erbium: YAG laser (Jennings et al., Lee SJ).
Dr Bettoli highlights that the classification of acne scars is difficult, even for acne experts.
An interesting study (Sewon et al.), on the classification of atrophic acne scars, is discussed. Based on this study, it is concluded that atrophic acne scars should be classified as icepick scars when <2mm, 2-4mm and >4mm. Therefore, the size is suggested to be considered as the primary identifying characteristic of scar classification systems.
Another prospective study on the evolution of scars is discussed (Tan et al.). Here it is concluded that 19.9% of patients reported resolution.
A study by Dréno et al. aimed to evaluate the efficacy of adapalene 0.3% benzoyl peroxide 2.5% gel in atrophic scar formation. This was found to be an effective treatment for moderate to severe acne vulgaris and a successful means of reducing existing scars 24 weeks post treatment.
Dr Bettoli explained that adapalene reduces inflammation by reducing TLRs activity. Adapalene 0.3% enhances collagen synthesis.
A multicenter, randomized, double-blind, vehicle-controlled study evaluated the efficacy and safety of trifarotene cream in the prevention of acne scarring. Trifarotene cream at 50μg/g daily was used (Schleicher et al.) and it showed to statistically significantly reduce the total number of acne-induced atrophic scars.
Speaker: Dr. PhD Øystein Grimstad (Tromsø, Norway)
Key points:
Dr Grimstad begins with a discussion on guidelines for the therapy of pathological scars (2020 update & position statement of the Brazilian expert group GREMCIQ). Glucocorticoids (triamcinolone) have strong consensus for treating hypertrophic scars and keloids via intralesional injections. In the case of hypertrophic scars and keloids, combining triamcinolone with cryosurgery is recommended. 5-Fluorouracil is recommended for the treatment of otherwise refractory hypertrophic scars and keloids (off label). It is also noted that there is no recommendation for verapamil.
The lack of updated international guidelines is attributed due to many factors such as; the heterogeneity of scars/ keloids, the timing of treatment, inconsistent interventions and more.
Dr Grimstad proceeds to discuss a scoping review on injection methods for intralesional corticosteroid administration for keloids (Yin et al.). This study found that the maximum dose per session varied between 20 – 80 mg, and the dosing per cm2 was between 1 – 20mg. Outcome measures varied, from height/ surface area/ Vancouver scar scale.
To understand the pathogenesis of pathological scars, we must consider; the role of immune cells (masT cells, macrophages, regulatory T-cells, dendritic cells), the aberrant wound healing process (prolonged inflammation, dysregulation of fibroblasts and collagen overproduction, abnormal extracellular matrix re-modelling), the signaling pathways involved (TGFβ/Smad, JAK/STAT, MAPK, PI3K/AKT) and lastly the role of genetics (IL-6 polymorphism, TGFβ receptor mutations).
Dr Grimstad then lists the main therapeutics used for keloids/ hypertrophic scars:
| ACE inhibitors – Captopril, Enalapril, Losartan | Anti-allergic agents – Tranilast |
| Antisense drugs – TGFβ1-antisense, SMAD3 antisense, hTERT antisense | Antiviral cytokines – Interferons |
| Ca2+-channel blockers - Verapamil | Chemotherapeutics – Bleomycin, Camptothecin, 5-Fluorouracil, Mitomycin C, Paclitaxel, Tamoxifen |
| Enzymes – Collagenase, Hyaluronidase | Vitamins – A, D3, E |
| Immunomodulators – Tacrolimus, Pimecrolimus | Monoclonal Abs – Dupilumab, αTGFβ1, αVEGFA |
| Neurotoxins – Botulinum A & E | Peripheral vasodilators – Pentoxifylline |
| Photosensitizer prodrug – 5-aminoleuvulinic acid | Plant based extracts – aloe vera, green tea, onion extracts, shikonin |
| Statins – Pravastatin | Steroids – Triamcinolone acetonide, Dexamethasone, Hydrocortisone acetate, Methylprednisolone |
There are numerous options for intralesional injection applications, including topical, patches, needle-free mechanisms, pneumatic jet-injection, fractional laser assisted, micro-needling prior to drug, microneedle patches, microneedle electroporation.
An intriguing systematic review on the recurrence and complications of peri-operative steroid injection of keloids (Zhang et al.) suggests that sessions of TAC 20 – 40mg/ml every 4 weeks, 1 – 2 weeks post-operatively, should be done.
A single-blinded, randomized trial compared intralesional triamcinolone and verapamil-triamcinolone injections in keloids (Haghani-Dogahe et al.) (given TAC 40mg/ml versus TAC 40mg/ml + Verapamil 2.5mg/ml). The conclusions highlighted the benefits of verapamil, as the combined treatment group proved more effective for keloids.
TCA & 5-FU treatment was found superior compared to TCA monotherapy for keloids and hypertrophic scars (Jianzhen Shi et al.).
For 5-FU, the typical concentration used is 1.5mg/ml – 50mg/ml in a dose of 0.02 – 0.4 ml/cm3 (max dose: 150mg/ treatment), every 1-4 weeks. Side effects may include pain on site, erythema, ulceration and hyperpigmentation.
For Botulinum toxin A, the typical concentration is 10 – 75 U/ml, at a dose of 2.5 – 5 U/cm3 (max 100 U/ treatment), every 4 – 12 weeks. Side effects include local temporary muscle weakness.
Speaker: Prof. Dr. Klaus Fritz (Landau, Germany)
Key points:
Prof. Fritz begins by outlining the main differences between a hypertrophic scar and a keloid. A hypertrophic scar can regress and is confined to the wound, whereas keloids do not regress and are not confined.
The S2k guidelines for the therapy of pathological scars (2020 update) is a key algorithm that is undoubtedly helpful for clinical practice. In summary, for keloids, if small, TAC and/ or cryosurgery are suggested, if treatment is refractory, then combination of TAC & cryosurgery and 5-FU OR surgery with follow-up, and if there is persistent erythema, pulsed dye laser is suggested.
For red scars, Prof. Fritz, suggests KTP, Pro yellow, pulsed dye laser & IPL.
A pulse width of 0.45ms of PDL proved more effective in decreasing scar size and improving scar pliability than that of 40ms, at 7J each (Manuskiatti W et al.). It was also shown that scar height and pliability were improved after 2 weeks and that improvement was higher with more visits (4 weeks interval).
For hypertrophic scar treatment with PDL the following are recommended (Manuskiatti W et al.): 1. Pulse duration: 0.45 – 1.5ms, 2. Fluence: 3 – 7 J/cm2, 4. Sufficient cooling, 5. Sessions: 4 – 8 in total, at 4 – 6 weeks apart.
The “gold standard” is the application of pulsed dye laser, on the day of removal of sutures, at 4.5J/cm2 in short pulse duration of 1.5 – 2ms. (Leclere FM et al.)
Prof. Fritz shared a study on the efficacy of PDL comparing to standard treatment (Waraphong M, Fitzpatrick) in which all scars showed significant flattening compared to control and intralesional treatments showed faster resolution than PDL. (the study used 585 nm flashlamp-pumped PDL, intralesional corticosteroid alone and intralesional corticosteroid with 5-FU)
Nd:Yag (1064nm) laser is suggested for extended scars and keloids. Prof. Fritz advices to start with intralesional NdYag, then to continue in less thick areas with topical laser and then PDL. The specificities for Nd:Yag, based on thickness are: for <0.5cm then PDL, for 0.5 – 1cm then PDL or Nd:Yag, lastly, if >1cm Nd:Yag is recommended.
In case of redness and hypopigmentation in scars, Prof. Fritz recommends ablation of the hypertrophic areas with Er:Yag, 2 PDL treatments, then 308nm Excimer for re-pigmentation, and a 18 treatments at 300 – 400mJ.
The S2k guidelines for the therapy of pathological scars (2020 update) in case of hypertrophic scars, direct clinicians based on whether or not tensile stress is present, if so, surgical relief via local flap surgery or graft is recommended. If not present, lasers, topicals, TAC and cryosurgery or compression are suggested.
Prof. Frtiz notes that for old scars, CO2 laser is an acceptable option, but for keloids a 95% reoccurrence after one year should be expected. (Nast, Fritz et al.)
In post-burn scars ablative and non-ablative lasers are gaining good ground as they provide good improvement on skin texture, thickness, contracture and more. (Beachkofsky et al.)
For non-ablative lasers for scars, targeting the dermis, Prof. Fritz suggests: 1064nm Nd:Yag, 1450nm, 1540nm Er:Glass and radiofrequency.
For inactive keloids, ablative fractional laser could be used in combination with steroids or for “laser-assisted drug delivery”.
For hyperpigmentation treatment, Q-Switched Nd:Yag and Picosecond are discussed, with the latter causing less thermal diffusion.
For hypopigmentation treatment, 308nm Excimer laser or light are suggested.
Prof. Fritz shares an interesting study on re-pigmentation by outer-root-sheath-derived melanocytes for vitiligo and leukoderma (Vanscheidt, Hunziker). Variable but stable re-pigmentation was recorded in all patients, showing that ORS-derived melanocytes are a promising technology to improving autologous melanocyte transplantation.
LLLT increases activity and migration of fibroblasts and macrophages, improving leucocyte mobility. (Jagdeo, Nguyen JK et al.). Also, Jadgdeo demonstrated that high fluence red or blue light inhibits fibroblast migration, which could lead to prevention of wound scarring.
Speaker: Prof. Dr. Dr. Klaus Eisendle (Bolzano, Italy)
Key points:
Dr Eisendle shares clinical cases in his engaging session. Scar complications such as ectropium and trapdoor or pincushing are discussed. Dr Eisendle highlights the importance of knowing when and how to plan appropriate grafts and flaps.
The surgical techniques of VY-plasty and Z-plasty are explained.
Dr Eisendle explains that VY-plasty is a V shaped incision with longitudinal tension, the tension causes the wound edges to come together. Y-shaped tension-free wound closure. Z-plasty is when two opposite transposition flaps are placed against each other.
Hypertrophic scars tend to be found when scars cross joints or skin folds at right angles, whereas keloids are mainly seen on the earlobe, shoulders, sternum.
The S2k guidelines shed light on radiotherapy for keloids. Adjuvant radiation after keloid excision is recommended when keloids tend to recur, and when keloids are large and difficult to treat otherwise.
HDR brachytherapy is recommended for adjuvant radiation therapy.
A recent study by Huang et al., recommends combining excision with radiotherapy or perioperative administration of steroids in order to improve the prognosis of earlobe keloids.



Bioderma Congress Reports EADV 2023

BIODERMA Congress Reports EADV Spring 2023

BIODERMA Congress Reports EADV 2022